-
生物活性
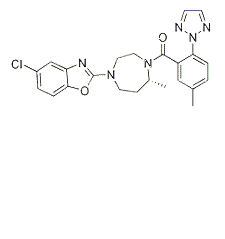
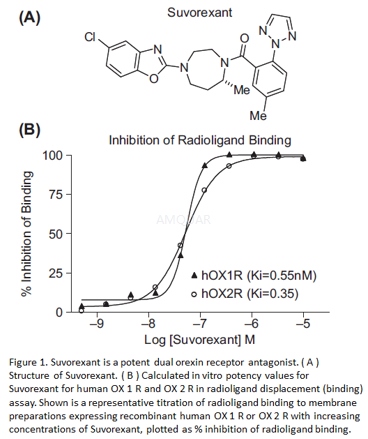
Suvorexant is a potent dual OX receptor antagonist with Ki of 0.55 nM and 0.35 nM for OX1 receptor and OX2 receptor, respectively. Suvorexant (MK-4305) is a first-in-class potentdual orexin receptor antagonist approved for the treatment of insomnia.
Orexin receptor activities of MK-4305
-
体外研究
-
体内研究
-
激酶实验
Radioligand Binding Assay[1]
Membranes were prepared from expressedhuman orexin 2 receptor (hOX2R) and the Ile408-Val variant of orexin 1 receptor(hOX1R) in CHO cells. Confluent CHO/OX2R and CHO/OX1R cells were dissociatedfrom flasks with PBS/1mMEDTA and centrifuged at 1000g for 10 min. The cell pelletswere homogenized with a Polytron in ice-cold 20mM Hepes, 1mMEDTA, at pH7.4, andcentrifuged at 20000g for 20min at 4 oC. This process was repeatedtwice. The final membrane pellet was resuspended at 5mg of membrane protein/mL inassay buffer (20mM Hepes, 125mM NaCl, 5mM KCl, pH 7.4). Bovine serum albuminwas added to achieve a final concentration of 1% and aliquots stored at -80oC.Radioligand binding assays were performed utilizing an automated Tecan Liquidhandling system and Packard Unifilter-96. Assays were performed at room temperaturein 96-well microtiter plates with a final assay volume of 1.0mL in 20mM Hepesbuffer (pH 7.4) containing 125nM NaCl and 5mM KCl. Solutions of test compoundswere prepared in DMSO and serially diluted with DMSO to yield 20μLof each of 10 solutions differing by 3-fold in concentration. Nonspecificbinding (NSB) is determined using a high-affinity ligand (1μM finalconcentration) and total binding (TB) is determined by using DMSO (2% finalconcentrations). A solution of receptor (30pM final, typically 2-10μgmembranes), and tritiated ligands (∼80Ci/mmole) were added to the test compounds.
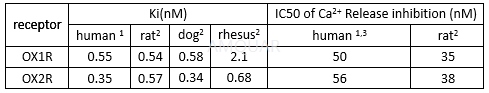
Orexin Receptor Binding[2]
Suvorexant binding to membranes preparedfrom Chinese hamster ovary (CHO) cells expressing either human OX1R or human OX2R was described above. Suvorexant binding to CHO cells expressing OX 1 R or OX2 R from rat, rhesus, and dog was evaluated using the same protocol. Briefly,Suvorexant – induced displacement of tritiated OX1R or OX2R ligands frommembranes was carried out in 20mM HEPES (pH 7.4), 120mM NaCl, 5mM KCl, and 5 mgmembrane protein for 120 minutes at room temperature. Nonspecific binding wasdetermined using unlabeled OX1R/OX2R antagonist at 1μ M, and K i valuesdetermined using the RAB Calc software package.
-
细胞实验
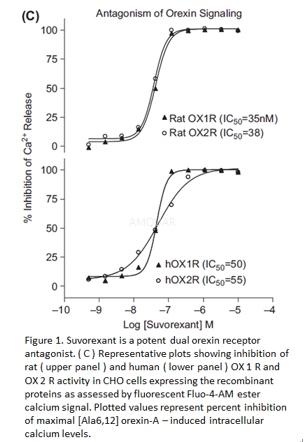
FLIPR Assay[1]
For intracellular calcium measurements, Chinesehamster ovary (CHO) cells expressing the Ile408-Val variant of the orexin 1receptor or the human orexin 2 receptor, were grown in Iscove’s modified DMEMcontaining 2mM L-glutamine, 0.5g/mL G418, 1% hypoxanthine-thymidine supplement,100U/mL penicillin, 100μg/mL streptomycin, and 10% heat-inactivated fetal calf serum. Thecells were seeded at 20000cells/well into Becton-Dickinson black 384-well clearbottom sterile plates coated with poly-D-lysine. All reagents were fromGIBCO-Invitrogen Corp. The seeded plates were incubated overnight at 37oCand 6% CO2. Ala-6,12 human orexin-A as the agonist was prepared as a0.5mM stock solution in 1% bovine serum albumin (BSA) and diluted in assaybuffer (HBSS containing 20mM HEPES and 2.5mM probenecid, pH 7.4) for use in theassay at a final concentration of 0.3-2nM. Test compounds were prepared as 10mMstock solution in DMSO, then diluted and pipetted in 384-well plates, first in DMSO,then assay buffer. On the day of the assay, cells were washed three times with100μL assay buffer and then incubated for 60 min (37oC, 6% CO2)in 60 μL of assay buffer containing 1 μMFluo-4AM ester, 0.02% pluronic acid,and 1% BSA. The dye loading solution was then aspirated and cells washed three timeswith 100μL of assay buffer. Then 30 μL of that same buffer is left in eachwell. Within the fluorescent imaging plate reader, test compounds were added tothe plate in a volume of 15μL, incubated for 5 min, and finally 15μL of agonistwas added. Fluorescence was measured for each well at 1 s intervals for 1 minand at 6 s intervals for 4 min, and the height of each fluorescence peak wascompared to the height of the fluorescence peak induced by 0.3-2nM Ala-6,12orexin-A with buffer in place of antagonist. For each antagonist, the IC50 value(the concentration of compound needed to inhibit 50% of the agonist response)was determined.
Orexin Receptor Inhibition of Activity[2]
Blockade of human OX1R and human OX2Ractivation by Suvorexant in CHO cells expressing these receptors was describedabove. In summary, calcium-dependent fluorescence of cells loaded withFluo-4-AM ester was evaluated following addition of modified human orexinpeptide ligand, [Ala6,12] orexin-A. Inhibition of this response was evaluatedusing increasing concentrations of antagonist delivered 5 min prior to[Ala6,12]orexin-A stimulation.
-
动物实验
Materials[4]
Suvorexant was suspended in 0.5% methylcellulose 400 just before its oral administration.
Experimentaldesign
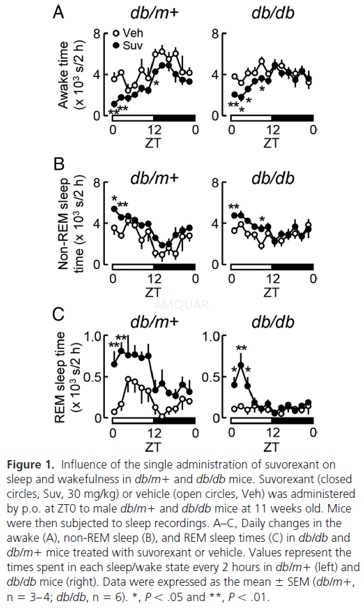
The effects of suvorexant on the states ofsleep and wakefulness were tested. Suvorexant (30mg/kg) or vehicle (0.5% methylcellulose 400) was administered by oral gavage (p.o.) to mice (males, 11wk old)once daily at ZT0 (ie, the beginning of the resting phase) for 2 weeks. Sleeprecordings were performed on days 1 and 7, as described below. On day 14, micefasted for 6 hours (atZT0–ZT6) were killed atZT6by cervical dislocation, andtissue samples were obtained for biochemical analyses.
Sleeprecordings
The states of sleep and wakefulness in micewere analyzed by a standard method, as follows. Under 3% isoflurane anesthesia,mice were implanted with electrodes for electroencephalogram (EEG) andelectromyogram (EMG) recordings. Regarding EEG, epidural electrodes were set byimplanting 4 stainless steel screws through holes drilled in the skull (~1 mmanterior to the bregma or lambda and 1.5 mm left- or right-lateral to themidline) with attaching to the dura mater. Regarding EMG, Teflon-coatedstainless steel wires were placed into the neck muscles bilaterally. After 3days of recovery, mice were moved to a recording cage in a sound-attenuatedroom. The electrodes were then connected to a data collection system through aswivel system that allowed the mice to move freely within the cage and take foodand water ad libitum. Mice were treated with suvorexant (30 mg/kg, p.o.) or vehicle,and EEG and EMG signals were recorded for 24 hours on experimental days 1 and7. The data collected were analyzed using Sleepsign software. The vigilance ofevery 4-second epoch was automatically classified into 3 stages, ie, wakefulness,REM, and non-REM sleep, according to standard criteria. Each automaticallydefined stage was reexamined visually, and corrected if necessary. Thevigilance states of 4-second epochs were assessed as follows: 1) wakefulnesswas defined by a high EMG amplitude, low EEG amplitude, and high Θ wave activity concomitant with the highest EMG values; 2) REM sleepwas defined by a low EMG amplitude, low EEG amplitude, and high Θ wave activity; and 3) non-REM sleep was defined by a low EMGamplitude, high EEG amplitude, and high δ waveactivity.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Cox CD, Breslin MJ, Whitman DB, et al. Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H -1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem. 2010;53(14):5320-5332.
[2] Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1-2):52-61.
[more]
分子式
C23H23ClN6O2 |
分子量
450.92 |
CAS号
1030377-33-3 |
储存方式
-20 ℃长期冷藏储存。冰袋运输 |
溶剂(常温)
|
DMSO
≥ 50 mg/mL |
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们