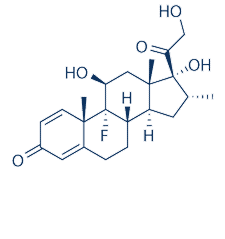
-
生物活性
An anti-inflammatory glucocorticoid. Inhibits the expression of the inducible but not the constitutive nitric oxide synthase in vascular endothelial cells (IC₅₀ = 5 nM). Enhances active cation transport in aortic smooth muscle cells by stimulating the Na+-K+ pump. Has anti-inflammatory and anti-rheumatic properties. Induces apoptosis in human thymocytes. In general, 500-1000 nM of dexamethasone is sufficient to induce apoptosis following a 6-hour incubation at 37 °C.
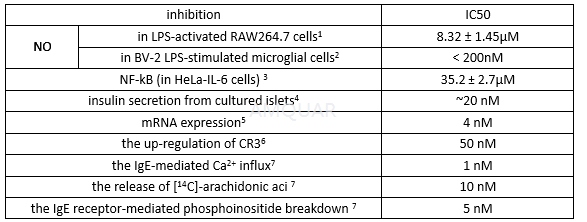
Dexamethasone increase ob gene expression with a EC50 of 10nM.[8]
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% propylene glycol
-
激酶实验
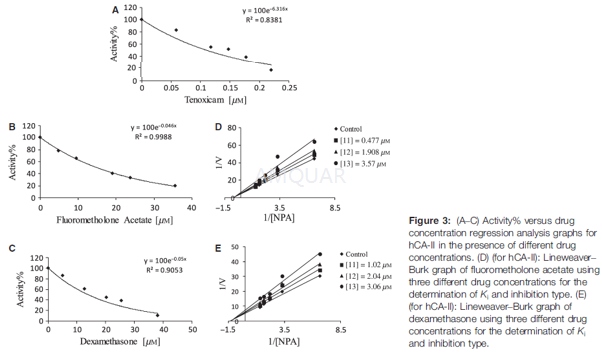
CA enzyme activity assay[9]
The activity of CA isozymes can be assayed by esterase activity that can be performed in vitro and followed spectrophotometrically at 348 nm. 4-nitrophenyl acetate (NPA) is the substrate in this method. We screened the conversion from 4-nitrophenyl acetate (NPA) to 4-nitrophenylate over a period of 3 min at 25 °C in the spectrophotometer. The enzymatic reaction contained 50 mM Tris–SO4 buffer (pH = 7. 4), 3 mM 4-nitrophenyl acetate, and enzyme solution in a total volume of 1 mL. A reference measurement was taken by the same cuvette without enzyme solution. The inhibitory effects of tenoxicam, fluorometholone acetate, and dexamethasone on the activity of purified carbonic anhydrase enzyme from human erythrocyte were tested. These experiments were performed three times for five different drug concentrations.
Control activity in the absence of drug was ascertained to be 100%. A percent activity versus drug concentration graph was plotted and IC50 values were calculated from these curves. Ki values of the drugs were calculated by measuring enzyme activity at three different drug concentrations with five different substrate concentrations. Ki constant and inhibition type Lineweaver–Burk curves were used.
-
细胞实验
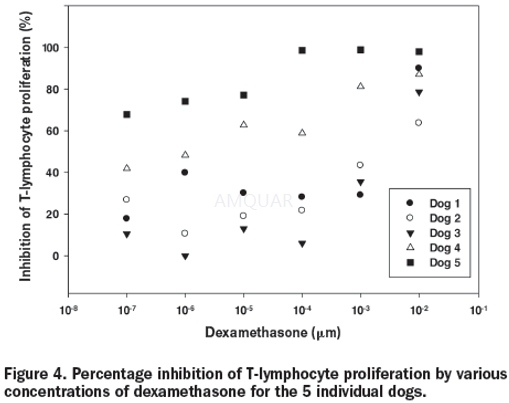
Cell proliferation and its suppression[10]
Whole blood was collected by jugular venipuncture into lithium heparinized tubes and processed within 1 h after collection, in healthy dogs. Density-gradient centrifugation with Histopaque 1077 was done to harvest peripheral blood mononuclear cells (PBMCs). A commercially available
cell-proliferation dye (CPD), eFluor 670, was used to assess lymphocyte proliferation. This fluorochrome-tagged dye binds to cellular proteins containing primary amines and is distributed equally to daughter cells upon division; thus, as cells divide, fluorescent staining becomes less bright. CPD eFluor 670 was added at concentrations of 5.0 μM to washed PBMCs (5 x 105cells). The cells were incubated for 10 min at 37°C in the dark, and labeling was stopped by the addition of 5 volumes giving (500 mL of cold complete RPMI (cRPMI) medium (RPMI 1640), with 10% fetal bovine serum (FBS), 5 mL of 1-M Hepes, 0.35 L of diluted b-mercaptoethanol, 5 mL of penicillin–streptomycin–glutamine, and 1.1 g of sodium bicarbonate. The cells were washed 3 times with cRPMI and transferred to 96-well platesin the presence of the T-cell mitogen concanavalin A , 5 mg/mL, and increasing concentrations of dexamethasone (0.1, 1, 10, 100, 1000, and 10 000μM). The plates were incubated at 37°C in humidified 5% CO2/95% air for 3 d before the cells were labeled for flow-cytometric analysis.
-
动物实验
Animals[11]
Female nu/nu nude mice (4–5 weeks old, 18–22 g) were obtained. The mice were housed under standard temperature (22–24 °C), humidity (50%–60%) and light (12 h light/12 h dark cycle) conditions with free access to food and water before being used in this study.
Tumor xenograft model
A549 cells (1×107cells, suspended in 200 μL of PBS) were inoculated subcutaneously into the right flank of nude mice. At approximately d 7, animals were grouped according to tumor volume, so that all groups had similar starting mean tumor volumes of approximately 100 mm3. The tumor diameter was measured with vernier calipers, and tumor volume was calculated by the following formula: tumor volume (mm3)=0.5×A(mm)×B(mm)2, where A is the larger diameter, and B is the smaller diameter.
Tumor growth inhibition assay
DEX and TAM(tamoxifen) were dissolved in 10% hydroxypropyl-β-cyclodextrin aqueous solution and 1,2-propanediol, respectively. DEX and TAM were administered by intragastric gavage every day. GEM was dissolved in a 0.9% sodium chloride solution and injected intravenously via a tail vein every 3 d. After being randomly divided into different groups with five mice per group, xenograft mice were treated with vehicle solution (as a blank control), TAM (50 mg/kg), GEM (20 mg/kg) and DEX (2, 4 mg/kg). Tumor measurements were taken every two days. After 32 d of treatment, the mice were euthanized by cervical displacement. All tumors and tissues were harvested after the final treatment for later assessment.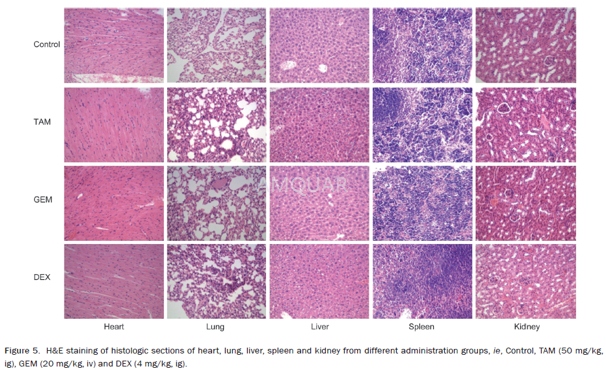
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Wang F, Yue Z, Xie P, et al. C19-Norditerpenoid Alkaloids from Aconitum szechenyianum and Their Effects on LPS-Activated NO Production. Molecules. 2016;21(9).
[2] Mateeva N, Gangapuram M, Mazzio E, Eyunni S, Soliman KF, Redda KK. Biological evaluation of synthetic chalcone and flavone derivatives as anti-inflammatory agents. Med Chem Res. 2015;24(4):1672-1680.
more
分子式
C22H29FO5 |
分子量
392.46 |
CAS号
50-02-2 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
25 mg/mL |
Water
Insoluble |
Ethanol
25 mg/mL |
体内溶解度
约20 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02864602 | Anesthesia, Conduction | Drug: IV dexamethasone | University Health Network, Toronto | Phase 3 | 2016-07-01 | 2016-08-11 |
| NCT01255358 | Nervous System Disorder|Genetic Syndrome | Drug: Dexamethasone | Erydel | Phase 2 | 2011-02-01 | 2011-12-27 |
| NCT00011362 | Infant, Newborn|Infant, Low Birth Weight|Infant, Small for Gestational Age|Infant, Premature|Bronchopulmonary Dysplasia | Drug: Dexamethasone Early|Drug: Dexamethasone Late | NICHD Neonatal Research Network|National Center for Research Resources (NCRR) | Phase 3 | 1992-09-01 | 2015-06-03 |
| NCT01892163 | Diabetic Macular Edema | Device: Ozurdex | Moorfields Eye Hospital NHS Foundation Trust|The Royal Wolverhampton Hospitals NHS Trust|Frimley Park Hospital NHS Trust|Brighton and Sussex University Hospitals NHS Trust|University Hospitals Bristol NHS Foundation Trust | Phase 3 | 2013-03-01 | 2017-01-03 |
| NCT02850601 | Lichen Planus | Drug: Dexamethasone | Brigham and Women's Hospital | Phase 2 | 2016-11-01 | 2016-07-29 |
| NCT02991339 | Liver Dysfunction|Hepatectomy|Bilirubinaemia|Jaundice | Drug: Dexamethasone | Shanghai Zhongshan Hospital | Phase 2|Phase 3 | 2016-06-01 | 2016-12-12 |
| NCT02402725 | Multiple Myeloma | Drug: Dexamethasone | Boston Medical Center | Phase 2 | 2015-05-01 | 2017-02-14 |
| NCT01925859 | Healthy | Drug: EryDex (dexamethasone sodium phosphate encapsulated erythrocytes) | Erydel | Phase 1 | 2013-06-01 | 2015-05-29 |
| NCT02322242 | Shoulder Surgery|Nerve Block | Drug: Systemic Dexamethasone|Drug: Perineural dexamethasone | Dr. Stephen Choi|The Physicians' Services Incorporated Foundation|Sunnybrook Health Sciences Centre | Phase 4 | 2015-01-01 | 2017-02-03 |
| NCT01284478 | Pseudophakic Cystoid Macular Edema,|Diabetic Macular Edema | Drug: Dexamethasone Implant | Northern California Retina Vitreous Associates|Allergan | Phase 2 | 2011-01-01 | 2012-10-03 |
| NCT01983449 | Peripheral Arterial Diseases | Drug: Dexamethasone Sodium Phosphate Injection, USP | Mercator MedSystems, Inc. | Phase 4 | 2013-11-01 | 2017-03-21 |
| NCT03043495 | Dexamethasone|Supraclavicular Brachial Plexus Block | Procedure: Ultrasound guided supraclavicular brachial plexus block|Drug: Dexamethasone | Eslam Ayman Mohamed Shawki|Cairo University | Phase 4 | 2016-10-01 | 2017-02-03 |
| NCT01372904 | Cisplatin Ototoxicity | Drug: Dexamethasone Phosphate | Meir Medical Center | Phase 4 | 2011-06-01 | 2014-07-16 |
| NCT03033316 | Multiple Myeloma | Drug: Dexamethasone | Enceladus Pharmaceuticals BV|University Hospital, Aachen|Accelovance | Phase 1|Phase 2 | 2017-01-01 | 2017-01-24 |
| NCT02815319 | Cancer | Drug: Dexamethasone | Ottawa Hospital Research Institute | Phase 4 | 2017-01-01 | 2017-02-21 |
| NCT01297010 | Vomiting Postoperative | Drug: Dexamethasone and ondasetron|Drug: Dexamethasone | Instituto Materno Infantil Prof. Fernando Figueira|Universidade Federal de Pernambuco | Phase 3 | 2011-03-01 | 2011-08-02 |
| NCT01094990 | Acute Leukemic Patients in Children | Drug: Dexamethasone | CHANCHAI TRAIVAREE|Phramongkutklao College of Medicine and Hospital | Phase 4 | 2011-04-01 | 2012-01-09 |
| NCT02399657 | Diabetes Mellitus|Macular Edema|Retinal Exudates and Deposits | Drug: Intravitreal dexamethasone 0.7mg implant | Inje University|Allergan | Phase 4 | 2015-02-01 | 2015-04-02 |
| NCT01731795 | Acute Respiratory Distress Syndrome | Drug: Dexamethasone | Dr. Negrin University Hospital|Fundacin Mutua Madrilea|Asociacin Cientfica Pulmn y Ventilacin Mecnica | Phase 4 | 2013-04-01 | 2016-07-12 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们