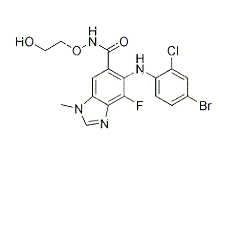
-
生物活性
Selumetinib (AZD6244; ARRY-142886) is an oral,non-ATP competitive, and highly selective MEK1 inhibitor(IC50 = 14 nM),
which is now in Phase 3 clinical trials fortreating different types of solid tumors. This compound inactivates the phosphorylation of ERK1/2 (IC50 < 40 nM). Selumetinib has little effect on p38, c-Jun-NH2-kinase, or the MEK/ERK5 pathways.
Selumetinibinhibits the high basal ERK1/2 phosphorylation in Malme-3M cells with an IC50of 10.3±2.0nM.[1]
Selumetinibinhibitsthe purified MEK1 with an IC50 of 14 ±0.79nM.[1]
Anti-proliferation of AZD6244
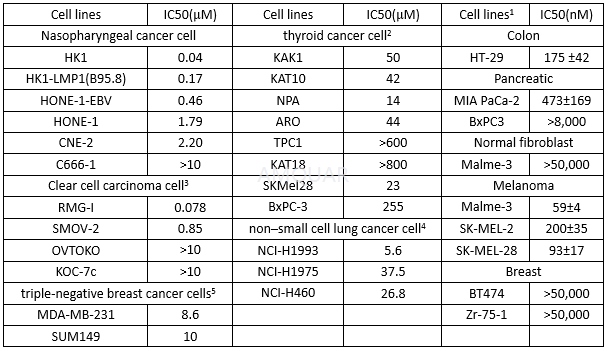
-
体外研究
-
体内研究
0.5% methylcellulose+0.2% Tween 80
-
激酶实验
Enzymatic assays[1]
NH2-terminal hexahistidine tagged,constitutively active MEK1 was expressed in baculovirus-infected Hi5 insectcells and purified by immobilized metal affinity chromatography, ion exchangeand gel filtration. The activity of MEK1 was assessed by measuring theincorporation of [γ-33P] phosphate from [γ-33P]ATP onto ERK2. The assay was carried out in a 96-well polypropylene plate withan incubation mixture (100μL) composed of 25mmol/L HEPES (pH 7.4), 10mmol/L MgCl2,5mmol/Lβ-glycerolphosphate, 100μmol/L sodium orthovanadate,5mmol/L DTT, 5nmol/L MEK1, 1μmol/L ERK2, and 0 to 80nmol/L compound (final concentration of 1%DMSO). The reactions were initiated by the addition of 10μmol/LATP(with 0.5μCi [γ-33P]ATP/well) and incubated at room temperature for 45min. An equal volume of 25% trichloracetic acid was added to stop the reactionand precipitate the proteins. Precipitated proteins were trapped onto glassfiber B filter plates, excess labeled ATP was washed off with 0.5% phosphoricacid, and radioactivity was counted in a liquid scintillation counter. ATP dependencewas determined by varying the amount of ATP in the reaction mixture. The datawere globally fitted using SigmaPlot. Values were calculated using thefollowing equation for noncompetitive inhibition: v = [VmaxxS /(1 +I / Ki)] / (Km + S).
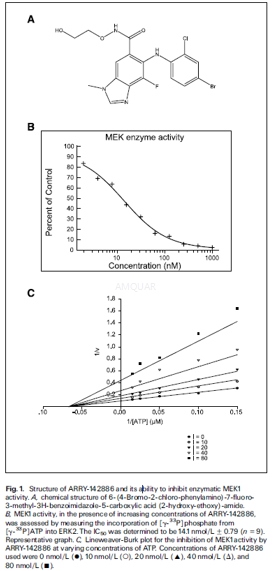
-
细胞实验
Cellculture and cell proliferation assay[6]
After planted in 96- or 6-well plates usingDMEM with 10 % fetal bovine serum (FBS) at 37 °C in humidified 5 % CO2,HCC1937 and MDAMB-231 cells were exposed for 24 h to various doses ofSelumetinib. For the transfected process, cells were with starved in DMEM withoutFBS for 6 h, then miR-302a-AMO, miR-302a-MIMIC, NC or pcDNA3.1-CUL1 were addedwith Lipofectamine 2000 Reagent following the manufacturer’s protocol. Cellproliferation assays were performed with tetrazolium salt (MTT) array accordingto the manufacturer’s protocol.
Evaluationof cell apoptosis by tunnel and FCM
For FCM detection, the procedures were sameas the cell culture previously. All cells of each group were collected andstained with Annexin V/PI following the instruction. The resulting was analyzedusing CellQuest software.
For tunnel test, the conditions were littledifferent. First, cells were cultured with or without IC50 Selumetinib. For therescue test, we first transferred the miR-302a AMO or pcDNA3.1-CUL1 or thenegative control for 6 h, and then we changed the medium which contain the10μMSelumetinib. Visualized apoptotic cells were labeled with the In Situ CellDeath Detection kit to detected positive ratio of terminaldeoxytransferasemediated dUTP-biotin nick end labelling (TUNEL) following tothe manufacturer’s recommendations.
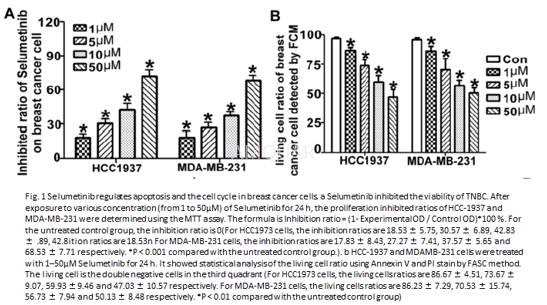
-
动物实验
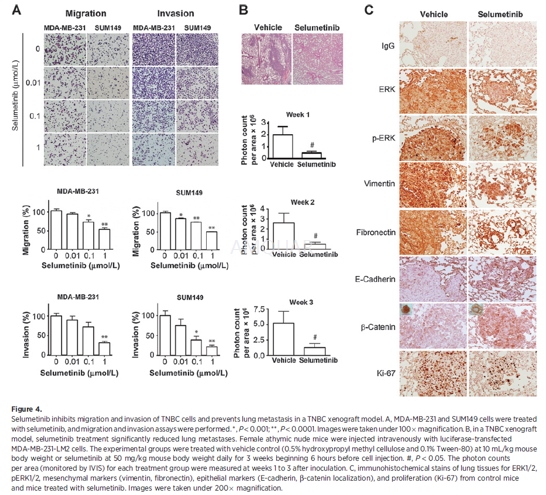
Selumetinibtreatment in a TNBC xenograft model[5]
Female athymic nu/nu mice, 6 to 8 weeks, averageweight 25 g, were randomly divided into two groups of 15 mice each. Suspensionsof MDA-MB-231-LM2, derivatives of MDA-MB-231 cells that were selected for itsability to metastasize to lung (luciferase-expressing cells), were resuspended(1 X 106 cells in 0.2 mL of PBS) and injected under asepticconditions into the tail vein of each mouse. Six hours before tumor cellinoculation, group 1 was given vehicle (0.5% hydroxypropyl methyl cellulose and0.1% Tween 80, 10 mL/kg mouse body weight) and group 2 was given selumetinib 50mg/kg mouse body weight by oral gavage. The treatment was continued daily withweekends off for 3 weeks. Mice were subjected to whole-body luciferase imaging underan IVIS 100 Imaging System at 1-week intervals for 3 weeks. Before imaging,mice were injected intraperitoneally with luciferin at 150 mg/kg body weight.Then mice were kept under anesthesia with isoflurane. Starting 5.5 minutesafter the luciferin injection, images were collected for 30 seconds each withmice in the ventral and dorsal positions. Images and amounts of bioluminescentsignals were analyzed using Living Image Software. At the end of theexperiment, tissue samples were collected and processed for expression oftarget proteins.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13(5):1576-1583.
[2] Ball DW, Jin N, Rosen DM, et al. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab. 2007;92(12):4712-4718.
[3] Bartholomeusz C, Oishi T, Saso H, et al. MEK1/2 inhibitor selumetinib (AZD6244) inhibits growth of ovarian clear cell carcinoma in a PEA-15-dependent manner in a mouse xenograft model. Mol Cancer Ther. 2012;11(2):360-369.
[more]
分子式
C17H15BrClFN4O3 |
分子量
457.68 |
CAS号
606143-52-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
90 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
10 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02768766 | Uveal Melanoma | Drug: Selumetinib | Richard D. Carvajal|AstraZeneca|Melanoma Research Alliance|Columbia University | Phase 1 | 2016-09-01 | 2016-12-15 |
| NCT01752569 | AIDS-related Kaposi's Sarcoma | Drug: Selumetinib | Sheffield Teaching Hospitals NHS Foundation Trust|University of Birmingham|AstraZeneca|Thermo Fisher Scientific|Cancer Research UK|University of Sheffield | Phase 1|Phase 2 | 2012-06-01 | 2014-09-18 |
| NCT01974349 | Solid Tumours | Drug: selumetinib (oral) | AstraZeneca | Phase 1 | 2013-11-01 | 2014-08-12 |
| NCT01931761 | Solid Tumours | Drug: [C14] selumetinib (oral) | AstraZeneca | Phase 1 | 2013-10-01 | 2015-06-29 |
| NCT02583542 | Triple-Negative Breast Cancer|Squamous Cell Lung Cancer|Non-squamous Cell Lung Cancer With KRAS Mutations|Non-squamous Cell Lung Cancer With Wild-type KRAS | Drug: AZD2014|Drug: AZD6244 | Queen Mary University of London|Cancer Research UK|AstraZeneca | Phase 1|Phase 2 | 2015-06-01 | 2015-10-20 |
| NCT01960374 | Healthy Volunteers Pharmacokinetic Study | Drug: Selumetinib | AstraZeneca | Phase 1 | 2013-10-01 | 2014-05-30 |
| NCT03040986 | KRAS NP_004976.2:p.G12R|Stage II Pancreatic Cancer|Stage IIA Pancreatic Cancer|Stage IIB Pancreatic Cancer|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer | Other: Laboratory Biomarker Analysis|Drug: Selumetinib Sulfate | National Cancer Institute (NCI) | Phase 2 | 2017-07-01 | 2017-02-09 |
| NCT02238782 | Healthy Volunteers Bioavailability Study | Drug: selumetinib 75mg single dose|Other: [14C] selumetinib IV solution | AstraZeneca | Phase 1 | 2014-10-01 | 2016-03-04 |
| NCT02046850 | Solid Tumours | Drug: selumetinib|Drug: rifampicin|Drug: selumetinib|Drug: rifampicin | AstraZeneca | Phase 1 | 2014-02-01 | 2014-04-28 |
| NCT01278615 | Recurrent Adult Diffuse Large Cell Lymphoma | Other: Laboratory Biomarker Analysis|Drug: Selumetinib | National Cancer Institute (NCI) | Phase 2 | 2010-12-01 | 2016-01-04 |
| NCT02063204 | Solid Tumours | Drug: selumetinib | AstraZeneca | Phase 1 | 2014-03-01 | 2015-06-29 |
| NCT01974752 | Metastatic|Uveal Melanoma | Drug: 75mg selumetinib|Drug: placebo|Drug: Dacarbazine | AstraZeneca | Phase 3 | 2014-04-01 | 2017-01-04 |
| NCT00588809 | Adult Acute Myeloid Leukemia With t(15;17)(q22;q12)|Adult Acute Promyelocytic Leukemia (M3)|Myelodysplastic Syndromes|Myelodysplastic/Myeloproliferative Neoplasms|Recurrent Adult Acute Myeloid Leukemia|Secondary Acute Myeloid Leukemia | Drug: selumetinib | National Cancer Institute (NCI) | Phase 2 | 2007-12-01 | 2015-07-06 |
| NCT02322749 | Healthy Volunteers Bioequivalence or Bioavailability Study | Drug: selumetinib 75mg single dose|Drug: selumetinib 75mg single dose|Drug: selumetinib 75mg single dose | AstraZeneca | Phase 1 | 2015-02-01 | 2016-07-20 |
| NCT01242605 | Biliary Tract Neoplasms|Cholangiocarcinoma|Gallbladder Neoplasms | Drug: selumetinib|Drug: gemcitabine|Drug: cisplatin | University College, London|AstraZeneca | Phase 1 | 2012-02-01 | 2016-05-11 |
| NCT00888134 | Adult Solid Neoplasm | Drug: Selumetinib | National Cancer Institute (NCI) | Phase 2 | 2009-07-01 | 2015-12-10 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们