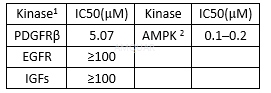
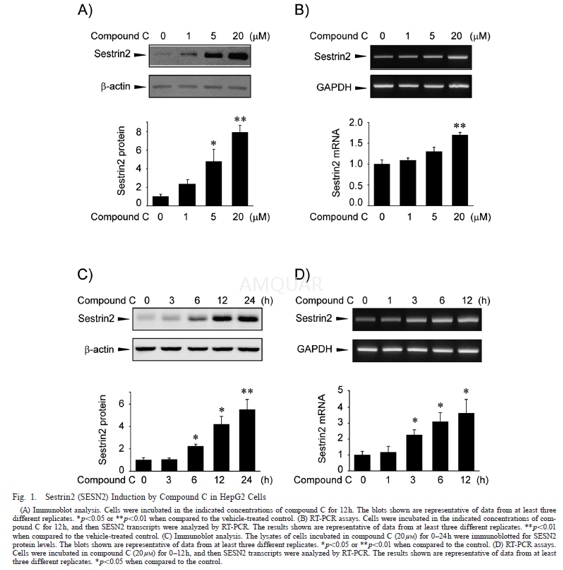
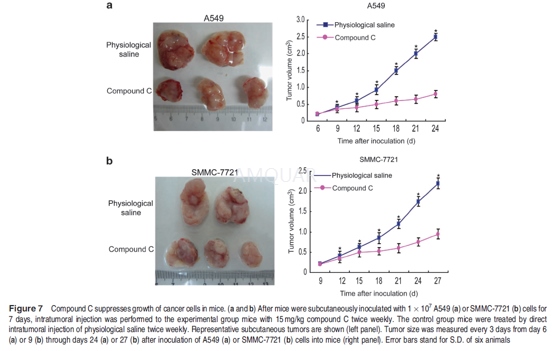
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01251562 | Solid Tumors | Drug: Sterile Compound c31510 for Injection | Berg, LLC | Phase 1 | 2011-01-01 | 2014-05-15 |
| NCT01607476 | Alzheimer's Disease | Drug: C11 PiB|Drug: F18 Flutametamol | Mayo Clinic | Phase 2 | 2012-07-01 | 2016-11-08 |
| NCT00811122 | Alzheimer's Disease | Radiation: 11C-PIB PET Scan|Radiation: FDG-PET Scan | University of Utah | Early Phase 1 | 2009-11-01 | 2016-08-09 |
| NCT01032031 | Skin Cancer | Dietary Supplement: Green tea + vitamin C high dose|Dietary Supplement: Placebo capsule | University of Manchester|University of Leeds|University of Bradford | | 2009-03-01 | 2016-03-07 |
| NCT02689843 | Polycystic Ovary Syndrome | Drug: Cyproterone compound + Spironolactone|Drug: Metformin|Drug: Pioglitazone | Shiraz University of Medical Sciences | Early Phase 1 | 2016-03-01 | 2016-08-02 |
| NCT02093169 | Healthy Men | Drug: Part A: Lu AF35700|Drug: Part B: Lu AF35700 | H. Lundbeck A/S | Phase 1 | 2014-02-01 | 2014-09-22 |
| NCT00704990 | Attention Deficit Hyperactivity Disorder | Dietary Supplement: Zinc, Magnesium, Vitamin B6, Vitamin C (Compound Natural Health Product for ADHD) | Kieran Cooley|The Canadian College of Naturopathic Medicine|University of Toronto|Centre for Addiction and Mental Health | Phase 2|Phase 3 | 2008-09-01 | 2015-10-15 |
| NCT00866853 | Hepatitis C|Viruses | Drug: TMC435 | Tibotec Pharmaceuticals, Ireland | Phase 1 | 2009-03-01 | 2010-04-26 |
| NCT00175695 | Pre-eclampsia | Drug: Recombinant human activated protein C or drotrecogin alpha | University of British Columbia | Phase 2 | 2004-12-01 | 2011-02-03 |
| NCT00908414 | HIV Infections | Drug: TMC589337 40 mg|Drug: TMC589337 100 mg|Drug: TMC589337 200 mg|Drug: TMC589337 400 mg|Drug: TMC589354 40 mg|Drug: TMC589354 100 mg|Drug: TMC589354 200 mg|Drug: TMC589354 400 mg|Drug: TMC589337 AA mg|Drug: TMC589354 BB mg|Drug: TMC589337 CC mg|Drug: TMC589354 DD mg|Drug: TMC589337 EE mg|Drug: TMC589354 YY mg|Drug: TMC310911 300 mg|Drug: TMC310911 600 mg|Drug: Placebo | Tibotec Pharmaceuticals, Ireland | Phase 1 | 2009-05-01 | 2016-10-27 |
| NCT02410525 | Healthy | Drug: [11C]PF-06427878|Drug: PF-06427878 10 mg|Drug: PF-06427878 600 mg | Pfizer | Phase 1 | 2015-05-01 | 2015-10-27 |
| NCT01063946 | Neoplasms, Malignant | Drug: Ombrabulin (AVE8062) | Sanofi | Phase 1 | 2010-01-01 | 2011-12-20 |
| NCT01022229 | Attention Deficit Hyperactivity Disorder | Dietary Supplement: Compound Natural Health Product|Dietary Supplement: Placebo | The Canadian College of Naturopathic Medicine|Health Canada|Centre for Addiction and Mental Health|SickKids Foundation | Phase 3 | 2013-11-01 | 2016-01-27 |
| NCT02120664 | Alzheimer's Disease | Drug: Florbetapir (18F)|Drug: 11C-PiB | Avid Radiopharmaceuticals | Phase 1 | 2014-04-01 | 2017-01-20 |
| NCT02564562 | Healthy | Drug: [14C] PF-06463922 | Pfizer | Phase 1 | 2015-10-01 | 2015-12-03 |
| NCT02240290 | N/A, as Healthy Volunteers | Drug: tozadenant tablet|Drug: C14-tozadenant capsule | Biotie Therapies Inc.|PRA Health Sciences|Tandem Labs|Xceleron Inc | Phase 1 | 2013-09-01 | 2016-01-13 |
| NCT02448004 | Healthy | Drug: JNJ-63623872 | Janssen Cilag N.V./S.A. | Phase 1 | 2015-05-01 | 2016-07-07 |
| NCT02211885 | Healthy | Drug: 14C-BIRB 796 BS | Boehringer Ingelheim | Phase 1 | 2002-10-01 | 2014-08-07 |
| NCT01961505 | Rheumatoid Arthritis | Drug: Compound tripterygium gel|Drug: Placebo | Guang'anmen Hospital of China Academy of Chinese Medical Sciences | Phase 2|Phase 3 | 2011-10-01 | 2013-10-09 |
| NCT01723553 | Atypical Alzheimers Disease|Logopenic Variant of Primary Progressive Aphasia (LPA)|Posterior Cortical Atrophy (PCA) | Drug: C-11 PiB | Mayo Clinic | Phase 1 | 2012-11-01 | 2015-12-18 |
| NCT01663389 | Infections, Bacterial | Drug: GSK1322322 1000 mg containing radioactive 14C-GSK1322322|Drug: GSK1322322 1200 mg containing radioactive 14C-GSK1322322 | GlaxoSmithKline | Phase 1 | 2012-08-01 | 2016-11-18 |
| NCT00910884 | Breast Cancer|Prostate Cancer|Sarcoma | Other: laboratory biomarker analysis|Procedure: therapeutic dietary intervention | Brabant Research | Phase 1 | 2016-04-01 | 2015-07-20 |
| NCT01279603 | Solid Tumors | Drug: GO-203-2c | Genus Oncology, LLC | Phase 1 | 2011-01-01 | 2014-09-18 |
| NCT00085917 | Hepatitis C|HIV Infections | Drug: Double dose pegylated interferon with weight based Ribavirin|Drug: standard dose pegylated interferon alfa -2a and ribavirin | National Institute of Allergy and Infectious Diseases (NIAID)|National Institutes of Health Clinical Center (CC) | Phase 2 | 2004-06-01 | 2011-06-21 |