-
生物活性
Sorafenib is a RAF kinase inhibitor which suppresses ERK phosphorylation. Studies indicate that sorafenib induces c-Raf phosphorylation at both Ser-43 and Ser-259. When combined with vitamin K1, phosphorylation is increased at these serine residues. Sorafenib also induces the phosphorylation of PKA. In addition, sorafenib induces c-Met phosphorylation at Tyr-1349, which consequently induces PI3K-Akt phosphorylation. Studies determined that sorafenib inhibits other kinases such as Flk-1 (VEGFR2), PDGFR (platelet-derived growth factor receptor), Flt-3/Flk-2 (FLT3), Ret, and c-Kit. Sorafenib is an inhibitor of Raf-1, Raf-B, PDGFR-βand Flt-4. Its ability to affect the Raf/Mek/Erk pathway makes it useful in cancer research studies.
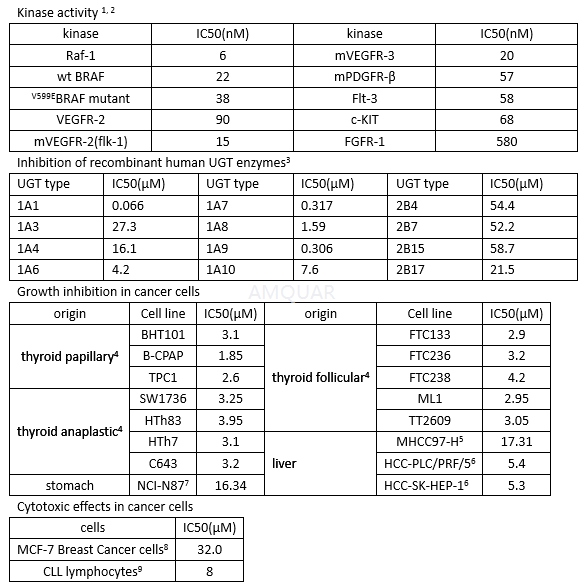
Sorafenib blocks RET/PTC3 autophosphorylation with an IC 50 of 47 nM, and inhibits the growth of cells carrying RET/V804L Mutants (IC 50 = 110 nM) or RET/V804M Mutants (IC 50 = 147 nM). [10]
-
体外研究
-
体内研究
-
激酶实验
In vitro Assays with Recombinant Raf-1 (Residues 305–648), BRAF (Residues 409–765), V599E BRAF (Residues 409–765), MEK-1, and Extracellular Signal-Regulated Kinase (ERK)-1. [2]
COOH-terminal kinase domains of Raf-1 (residues 305–648) and BRAF (residues 409–765) were generated by PCR. The BRAF (residues 409–765) V599E mutation was introduced using the Quik Change Site-directed Mutagenesis kit according to the manufacturer’s protocol. Recombinant baculoviruses expressing Raf-1 (residues 305–648), BRAF (residues 409–765), and V599E BRAF (residues 409–765) were purified as fusion proteins. Full-length human MEK-1 was generated by PCR and purified as a fusion protein from Escherichia coli lysates.
To test compound inhibition against various RAF kinase isoforms, BAY 43-9006 was added to a mixture of Raf-1 (80ng), wt BRAF, or V599E BRAF (80ng) with MEK-1 (1μg) in assay buffer [20mmol/L Tris (pH 8.2), 100mmol/L NaCl, 5mmol/L MgCl2, and 0.15%β-mercaptoethanol] at a final concentration of 1% DMSO. The RAF kinase assay (final volume of 50μL) was initiated by adding 25μL of 10μmol/LƳ-[33P] ATP (400Ci/mol) andincubated at 32°C for 25 minutes. Phosphorylated MEK-1 was harvested by filtration onto a phosphocellulose mat, and 1% phosphoric acid was used to wash away unbound radioactivity. After drying by microwave heating, aβ-plate counter was used to quantify filter-bound radioactivity. Activated MEK-1 and ERK-1 were purchased and assayed according to manufacturer’s instructions.
In Vitro Kinase Assays[10] For the in vitro RET autophosphorylation assay, subconfluent NIH3T3 cells stably transfected with RET/PTC3 were solubilized in lysis buffer without phosphatase inhibitors (sodium fluoride, sodium pyrophosphate, and sodium vanadate). Then, 200 μ g of proteins were immune-precipitated with anti-RET; immune-complexes were recovered with protein A – Sepharose beads, washed five times with kinase buffer (20mM HEPES at pH 7.5, 150mM NaCl, 10% glycerol, 0.1% Triton X-100,
15mM MnCl2, and 15mM MgCl2) and incubated 20 minutes at room temperature in kinase buffer containing 2.5 μCi of [ γ-32P]ATP and unlabeled ATP (20μ M). Samples were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were dried and exposed to film for autoradiography. Signal intensity was analyzed using a PhosphorImager (Typhoon 8600) interfaced with the Image Quant software. For phosphorylation of the synthetic substrate, RET immune-complexes were incubated (20 minutes at room temperature) in kinase buffer containing 200μM poly-(L-glutamic acid-L-tyrosine [poly-GT]), 2.5 μCi of [γ-32P] ATP, and unlabeled ATP (20μ M). Samples were spotted on Whatman 3MM paper, and32P incorporation was measured with a beta counter scintillator. Each experiment was performed at least three times.
-
细胞实验
Preparation of Sorafenib[1]
Sorafenibis dissolved in DMSO for in vitro experiments.
Cell Lines, Reagents, and Western Blot Analysis
The MDA-MB-231 human mammary adenocarcinoma cell line was obtained. Cell lines were maintained in DMEM (GIBCO), supplemented with 1% L-glutamine (GIBCO), 1% HEPES buffer (GIBCO), and 10% heat-inactivated fetal bovine serum (FBS). Cells were plated at 200,000 cells per well in 12-well tissue culture plates in growth media and incubated overnight. Media was removed and replaced with DMEM supplemented with 0.1% BSA containing either various concentrations of sorafenib, U0126, or vehicle (DMSO) for 2 h. Cells were washed with cold PBS containing 0.1 mM vanadate and lysed in a 1% Triton X-100 solution containing protease inhibitors. Lysates were clarified by centrifugation, subjected to SDS-PAGE, transferred to nitrocellulose membranes, and blocked for 1 h in TBS containing 5% non-fat dry milk and 1% BSA. Membranes were probed for 1 h with antibodies to pMEK1/2 (ser217/ ser221), MEK1/2, pERK1/2 (thr202/tyr204), ERK1/2, pPKB (ser473), and PKB. The antibodies were used at a dilution of 1:000. Blots were developed with horseradish peroxidase (HRP)–conjugated secondary antibodies and Amersham ECL reagent on Amersham Hyperfilm.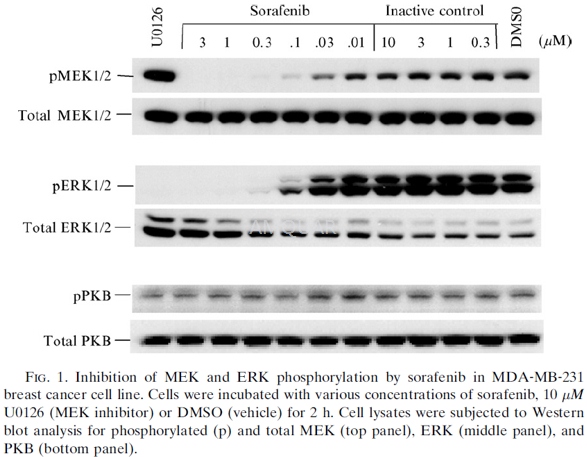
-
动物实验
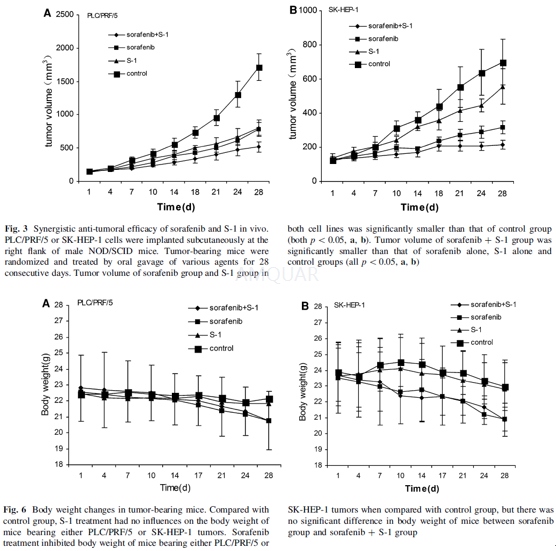
Tumoricidal efficacy of sorafenib and S-1 against HCC in animal model[6]
Male NOD/SCID mice (5–6 weeks old) werepurchased and kept in a SPF environment. The mice were exposed to a 12:12-hlight–dark cycle and fed with food and water ad libitum in a barrier facility. NOD/SCIDmice were inoculated subcutaneously at right flank with PLC/PRF/5 or SK-HEP-1cells (1 x 107cells/mouse). Mice were randomized into four groups (6mice per group) when tumors reached up to a volume of 120–150 mm3.Treatment for 4 groups was as follows: daily oral gavage of 10 mg/kg of S-1(thepro-drug of 5-FU) alone (S-1 group); 10 mg/kg ofsorafenib alone (sorafenib group); combination of S-1 and sorafenib (both 10mg/kg) (combined group); 0.2 ml normal saline (control group). Tumor length (L)and width (W) were measured with a caliper twice per week, and tumor volume wascalculated according to the formula 0.52 x L x W2. Body weight ofmice was measured simultaneously.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43‐9006, Nexavar®), a Dual‐Action Inhibitor That Targets RAF/MEK/ERK Pathway in Tumor Cells and Tyrosine Kinases VEGFR/PDGFR in Tumor Vasculature. 2006;407:597-612.
[2] Wilhelm SM CC, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004 64(19):7099-7109.
more
分子式
C21H16ClF3N4O3 |
分子量
464.82 |
CAS号
284461-73-0 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
20 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02122003 | Metastatic Renal Cell Carcinoma | Drug: Sorafenib | Fondazione IRCCS Istituto Nazionale dei Tumori, Milano | Phase 2 | 2014-05-01 | 2014-04-22 |
| NCT01425216 | Keloids | Drug: Sorafenib | Tirgan, Michael H., M.D. | Phase 2 | 2013-03-01 | 2016-10-17 |
| NCT00478114 | Renal Cell Carcinoma | Drug: sorafenib | Mahidol University|Bayer | Phase 3 | 2007-05-01 | 2011-08-25 |
| NCT02636426 | Neoplasms | Drug: sorafenib | VU University Medical Center | Phase 1 | 2015-09-01 | 2017-03-17 |
| NCT02288507 | Hepatocellular Cancer | Drug: Sorafenib|Radiation: yttrium-90 radioembolization | University of Hawaii | Phase 1 | 2014-11-01 | 2014-11-10 |
| NCT00780169 | Metastatic Colorectal Cancer | Drug: sorafenib + FOLFIRI | Ottawa Hospital Research Institute|Bayer | Phase 1 | 2008-10-01 | 2013-05-09 |
| NCT00544609 | Bladder Cancer | Drug: Sorafenib|Procedure: Radiotherapy | Spanish Oncology Genito-Urinary Group | Phase 1 | 2007-12-01 | 2014-10-01 |
| NCT02084732 | Thyroid Cancer | Drug: Sorafenib | Instituto Nacional de Cancerologia, Columbia | Phase 2 | 2013-10-01 | 2016-08-16 |
| NCT00875745 | Leukemia, Myeloid, Acute|Leukemia, Promyelocytic, Acute|Myelodysplastic Syndromes | Drug: Sorafenib-Vorinostat | Indiana University School of Medicine|Bayer|Indiana University | Phase 1 | 2009-04-01 | 2014-09-17 |
| NCT00997022 | Hepatocellular Cancer | Drug: Sorafenib | Columbia University|Bayer|Onyx Pharmaceuticals | Phase 1 | 2009-05-01 | 2015-08-07 |
| NCT00480389 | Renal Cell Carcinoma|Metastatic Disease | Drug: Sorafenib | University Health Network, Toronto|Bayer | Phase 2 | 2007-05-01 | 2015-12-07 |
| NCT02733809 | Hepatocellular Carcinoma | Drug: Sorafenib | King Saud University|King Faisal Specialist Hospital & Research Center | Phase 4 | 2014-01-01 | 2016-04-05 |
| NCT02021929 | Hepatopulmonary Syndrome | Drug: Sorafenib|Drug: Placebo | University of Pennsylvania|National Heart, Lung, and Blood Institute (NHLBI) | Phase 2 | 2014-03-01 | 2016-07-05 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们