-
生物活性
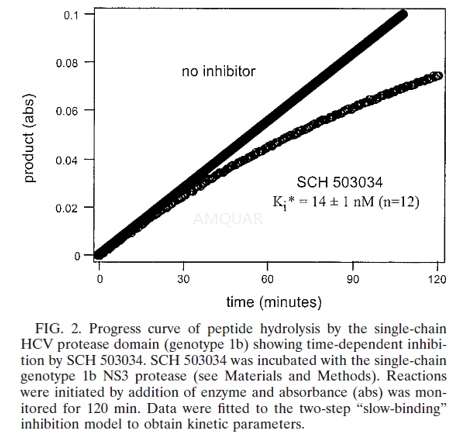
Boceprevir is a potent inhibitor in both enzyme assay (Ki* = 14 nM) and cell-based replicon assay (EC 90 = 0.35 microM). It is highly selective (2200x) against human neutrophil elastase (HNE). Boceprevir is well tolerated in humans and demonstrated antiviral activity in phase I clinical trials. It is currently in phase II trials. This Account details the complexity and challenges encountered in the drug discovery process. Boceprevir, an HCV NS3/4A protease inhibitor, is the first directagent approved for treatment of hepatitis C infection.
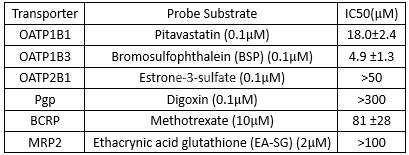
Boceprevirinhibits NS3 protease with a Ki value of 14nM and the replicon with an EC90 of350 nM.[1]
Potencyof boceprevir against HCV
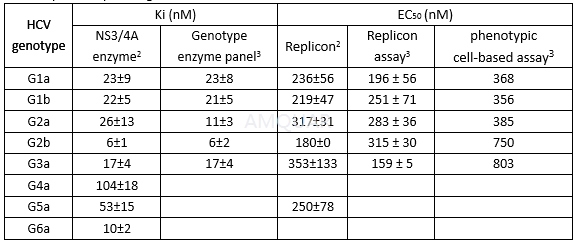
Invitro evaluation of boceprevir as an inhibitor of several uptake and effluxtransporters[4]
-
体外研究
-
体内研究
-
激酶实验
NS3enzyme assay
Single-chain NS3 protease inhibition wasevaluated using the chromogenic assay. The assays were performed at 30°C in a96-well microtiter plate. One-hundred microliters protease (3 to 5nM nominally)was added to 100μl of assay buffer (25mM morpholinepropanesulfonic acid, pH 6.5, 20%glycerol, 0.3M NaCl, 0.05% lauryl maltoside, 5μM EDTA, 5μM dithiothreitol)containing chromogenic substrate acetyl-DTEDVVP(norvaline)-Ophenylazophenol. Thereactions were monitored at an interval of 30s for 1h for change in absorbanceat 370nm using a Spectromax Plus microtiter plate reader.
-
细胞实验
HCVreplicon inhibition
Cells were seeded at 7,000 cells per wellof a 96-well plate in DMEM with 2% FBS and without G418. The compound undertest was solubilized in 100% DMSO and was added to the wells as a 10-point two-or three fold dilution series to a final DMSO concentration of 0.5% (vol/vol).The cells were incubated at 37°C in 5% CO2 for 3 days. After 3 days, the mediumwas removed and the total RNA was extracted by using the RN easy 96 kit. The extractedRNA was eluted in nuclease-free water; and the amounts of HCV RNA, rRNA, andglyceraldehyde-3-phosphate dehydrogenase (GAPDH) RNA were determined byqRT-PCR.
qRT-PCR
qRT-PCR was performed with an ABI Prism7900HT sequence detection system. Briefly, 18S rRNA and HCV replicon RNA werequantified in a single-step duplexed reaction with rRNA predevelopment reagentand primers and probe specific for the neomycin phosphotransferase gene,respectively. The RT reaction was carried out at 48 °C for 30 min, followed by adenaturation step at 95 °C for 10 min. The PCR amplification was conducted in 40cycles, each of which was 95 °C for 15 s followed by 60°C for 1 min. The amountof GAPDH RNA was determined in a separate reaction by using the GAPDHpredevelopment reagent mix (Applied Biosystems). Total RNA extracted from replicon-bearingcells was used to construct standard curves. HCV RNA copies were quantified bythe National Genetics Institute, and the total RNA concentration was determinedby UV spectrophotometry. The amount of HCV RNA, 18S rRNA, or GAPDH RNA in eachsample was determined by comparison to the standard curves and was expressed asthe number of HCV RNA copies per g of total RNA (by using rRNA as a marker fortotal RNA measurement) or the amount of GAPDH RNA relative to the amount oftotal RNA.
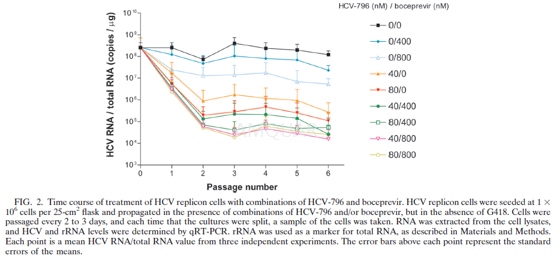
-
动物实验
Rat, Dog, and Monkey Pharmacokinetic Studies[6]
For rat, dog and monkey studies, Boceprevirdosing solutions were prepared freshly in 20%
hydroxylpropyl-β-cyclodextran for intravenous studies or in 0.4% methylcellulose fororal studies. For IV studies male Spraque-Dawly rats (~250 gm, Charles River,Wisconsin) were surgically prepared with jugular vein and femural artery cathetersat least two days prior to the dosing. The IV dose was administered via thejugular vein as a bolus dose. Blood samples (0.5 ml) were collected through thefemoral artery. The oral dose was administered by gavage. Blood samples werecollected from the femural vein. Dogs and monkeys were dosed intravenous at 3mg/kg.Serial blood samples (2 ml) from dogs and monkeys were collected from temporaryindwelling saphenous vein catheters or by venipuncture of the cephalic vein.The oral dose was administered to dogs or monkeys by gavage. All blood samples weretransferred to heparin tubes, mixed, and centrifuged. Plasma samples werestored at -80 oC before analysis.
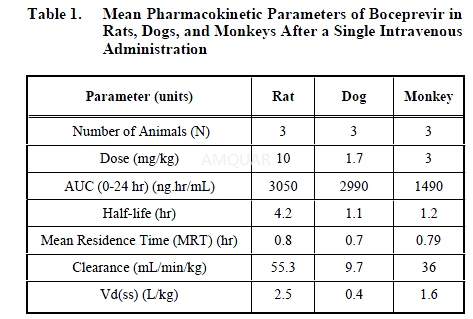
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Rotella DP. The discovery and development of boceprevir. Expert Opin Drug Discov. 2013;8(11):1439-1447.
[2] Howe AY, Venkatraman S. The Discovery and Development of Boceprevir: A Novel, First-generation Inhibitor of the Hepatitis C Virus NS3/4A Serine Protease. J Clin Transl Hepatol. 2013;1(1):22-32.
[more]
分子式
C27H45N5O5 |
分子量
519.68 |
CAS号
394730-60-0 |
储存方式
-20 ℃长期冷藏储存。冰袋运输 |
溶剂(常温)
|
DMSO
≥ 10 mg/mL |
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们