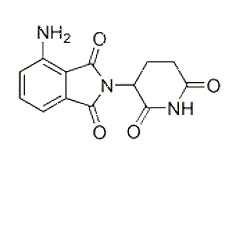
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01177735 | Multiple Myeloma | Drug: Pomalidomide | University of Arkansas|Celgene Corporation | Phase 2 | 2011-10-01 | 2015-02-18 |
| NCT01324947 | Multiple Myeloma | Drug: pomalidomide | Celgene Corporation | Phase 3 | 2011-03-01 | 2016-10-28 |
| NCT01198067 | Lymphoma|Myeloma | Drug: Pomalidomide|Drug: Pomalidomide | M.D. Anderson Cancer Center|Celgene Corporation | Phase 1 | 2010-10-01 | 2016-11-28 |
| NCT00717522 | Soft Tissue Sarcoma | Drug: Pomalidomide | Celgene Corporation|Celgene | Phase 2 | 2008-08-01 | 2013-03-06 |
| NCT01053949 | Multiple Myeloma | Drug: Pomalidomide|Drug: Pomalidomide | University Hospital, Lille|Celgene Corporation | Phase 2 | 2009-10-01 | 2015-11-03 |
| NCT01997840 | Multiple Myeloma | Drug: ACY-1215 (Ricolinostat) in combination with pomalidomide and dexamethasone | Acetylon Pharmaceuticals Incorporated | Phase 1|Phase 2 | 2013-11-01 | 2016-10-17 |
| NCT01522547 | Anemia, Sickle Cell | Drug: pomalidomide | Celgene Corporation|Celgene | Phase 1 | 2007-08-01 | 2013-12-24 |
| NCT02164955 | Multiple Myeloma | Drug: IMNOVID | Celgene | | 2014-06-01 | 2017-02-01 |
| NCT00949364 | Myeloproliferative Neoplasms | Drug: Pomalidomide | University of Ulm | Phase 2 | 2009-12-01 | 2013-09-09 |
| NCT02045017 | Multiple Myeloma | Drug: Pomalidomide and Dexamethasone | Celgene Corporation | Phase 2 | 2014-05-01 | 2016-09-12 |
| NCT01734928 | Multiple Myeloma | Drug: Pomalidomide|Drug: Bortezomib|Drug: Dexamethasone | Celgene | Phase 3 | 2013-01-07 | 2017-02-28 |
| NCT01078974 | Waldenstrom's Macroglobulinemia | Drug: pomalidomide|Drug: dexamethasone|Drug: rituximab | Steven P. Treon, MD, PhD|Celgene Corporation|Dana-Farber Cancer Institute | Phase 1 | 2010-05-01 | 2016-06-13 |
| NCT01474330 | Healthy | Drug: Pomalidomide|Drug: Placebo | Celgene Corporation | Phase 1 | 2011-08-01 | 2016-03-25 |
| NCT01568294 | Multiple Myeloma | Drug: pomalidomide | Celgene Corporation|Celgene | Phase 1 | 2012-04-01 | 2015-08-19 |
| NCT01178281 | Primary Myelofibrosis | Drug: Pomalidomide 0.5 mg|Drug: Placebo | Celgene | Phase 3 | 2010-09-01 | 2017-02-01 |
| NCT01311687 | Multiple Myeloma | Drug: pomalidomide|Drug: dexamethasone | Celgene | Phase 3 | 2011-03-01 | 2017-01-19 |
| NCT01979276 | Multiple Myeloma | Drug: Romidepsin|Drug: pomalidomide|Drug: Dexamethasone | Weill Medical College of Cornell University|Celgene Corporation | Phase 1|Phase 2 | 2013-11-01 | 2017-02-07 |
| NCT02555839 | Multiple Myeloma | Drug: Pomalidomide|Drug: Dexamethasone | Celgene | | 2015-03-01 | 2017-03-02 |
| NCT01541332 | Multiple Myeloma | Drug: Pomalidomide|Drug: Pegylated Liposomal Doxorubicin (PLD)|Drug: Dexamethasone | Oncotherapeutics|Celgene Corporation | Phase 1|Phase 2 | 2012-02-01 | 2016-03-08 |
| NCT00946270 | Polycythemia Vera|Thrombocythemia | Drug: CC-4047|Drug: Prednisone | M.D. Anderson Cancer Center|Celgene | Phase 2 | 2009-07-01 | 2016-11-14 |
| NCT01495598 | Kaposi Sarcoma|Sarcoma, Kaposi | Drug: Pomalidomide | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1|Phase 2 | 2011-12-09 | 2017-01-24 |
| NCT02011113 | Multiple Myeloma | Drug: Pomalidomide|Drug: Dexamethasone | Celgene Corporation | Phase 2 | 2013-12-01 | 2016-09-12 |