| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00741403 | Advanced Cancer|Metastatic Cancer|Lymphoma|Solid Tumors|Advanced Malignancies | Drug: CPI-613 | Cornerstone Pharmaceuticals, Inc. | Phase 1 | 2008-08-01 | 2016-12-28 |
| NCT01520805 | Acute Myeloid Leukemia (AML)|Myelodysplastic Syndrome (MDS) | Drug: CPI-613 | Cornerstone Pharmaceuticals, Inc. | Phase 2 | 2016-01-01 | 2016-12-27 |
| NCT01832857 | Cancer | Drug: CPI-613 | Cornerstone Pharmaceuticals, Inc. | Phase 2 | 2013-06-01 | 2016-12-27 |
| NCT01034475 | Advanced Hematologic Malignancies | Drug: CPI-613 | Wake Forest University Health Sciences | Phase 1 | 2010-03-01 | 2017-01-17 |
| NCT00907166 | Cancer|Pancreatic Cancer|Pancreatic Carcinoma | Drug: CPI-613|Drug: CPI-613|Drug: Gemcitabine|Drug: Gemcitabine|Drug: Gemcitabine | Cornerstone Pharmaceuticals, Inc. | Phase 1|Phase 2 | 2009-05-01 | 2017-03-19 |
| NCT01768897 | Adult Acute Myeloid Leukemia With 11q23 (MLL) Abnormalities|Adult Acute Myeloid Leukemia With Del(5q)|Adult Acute Myeloid Leukemia With Inv(16)(p13;q22)|Adult Acute Myeloid Leukemia With t(15;17)(q22;q12)|Adult Acute Myeloid Leukemia With t(16;16)(p13;q22)|Adult Acute Myeloid Leukemia With t(8;21)(q22;q22)|Recurrent Adult Acute Myeloid Leukemia | Drug: CPI-613|Drug: cytarabine|Drug: mitoxantrone hydrochloride|Other: laboratory biomarker analysis|Other: pharmacological study | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2013-01-01 | 2016-12-23 |
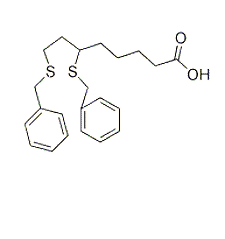
| NCT01931787 | Recurrent Small Cell Lung Cancer | Drug: 6,8-bis(benzylthio)octanoic acid | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2013-10-01 | 2016-12-23 |
| NCT01830322 | Metastatic Pancreatic Adenocarcinoma | Drug: CPI-613|Drug: Gemcitabine|Drug: Any non-gemcitabine chemotherapies or best supportive care | Cornerstone Pharmaceuticals, Inc. | Phase 2 | 2014-01-01 | 2013-08-13 |
| NCT02168140 | Adult Lymphocyte Depletion Hodgkin Lymphoma|Adult Lymphocyte Predominant Hodgkin Lymphoma|Adult Mixed Cellularity Hodgkin Lymphoma|Adult Nasal Type Extranodal NK/T-cell Lymphoma|Adult Nodular Sclerosis Hodgkin Lymphoma|Anaplastic Large Cell Lymphoma|Angioimmunoblastic T-cell Lymphoma|Hepatosplenic T-cell Lymphoma|Noncutaneous Extranodal Lymphoma|Peripheral T-cell Lymphoma|Recurrent Adult Acute Lymphoblastic Leukemia|Recurrent Adult Hodgkin Lymphoma|Recurrent Adult T-cell Leukemia/Lymphoma|Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|T-cell Adult Acute Lymphoblastic Leukemia|T-cell Large Granular Lymphocyte Leukemia | Drug: 6,8-bis(benzylthio)octanoic acid|Drug: bendamustine hydrochloride | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2014-09-01 | 2017-03-13 |
| NCT01902381 | Previously Treated Myelodysplastic Syndromes | Drug: 6,8-bis(benzylthio)octanoic acid | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 2 | 2013-08-01 | 2017-01-17 |
| NCT02168907 | B-cell Adult Acute Lymphoblastic Leukemia|B-cell Chronic Lymphocytic Leukemia|Cutaneous B-cell Non-Hodgkin Lymphoma|Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Intraocular Lymphoma|Nodal Marginal Zone B-cell Lymphoma|Recurrent Adult Acute Lymphoblastic Leukemia|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Grade III Lymphomatoid Granulomatosis|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Refractory Hairy Cell Leukemia|Small Intestine Lymphoma|Splenic Marginal Zone Lymphoma|Testicular Lymphoma|Waldenstr枚m Macroglobulinemia | Drug: 6,8-bis(benzylthio)octanoic acid|Drug: bendamustine hydrochloride|Biological: rituximab|Other: laboratory biomarker analysis | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2014-12-01 | 2017-02-20 |
| NCT02232152 | Mucinous Adenocarcinoma of the Colon|Mucinous Adenocarcinoma of the Rectum|Recurrent Colon Cancer|Recurrent Rectal Cancer|Signet Ring Adenocarcinoma of the Colon|Signet Ring Adenocarcinoma of the Rectum|Stage IIIA Colon Cancer|Stage IIIA Rectal Cancer|Stage IIIB Colon Cancer|Stage IIIB Rectal Cancer|Stage IIIC Colon Cancer|Stage IIIC Rectal Cancer|Stage IVA Colon Cancer|Stage IVA Rectal Cancer|Stage IVB Colon Cancer|Stage IVB Rectal Cancer | Drug: 6,8-bis(benzylthio)octanoic acid|Drug: fluorouracil|Other: pharmacological study|Other: laboratory biomarker analysis | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2014-12-01 | 2017-03-13 |
| NCT01766219 | Adult Primary Cholangiocellular Carcinoma|Advanced Adult Primary Liver Cancer|Cholangiocarcinoma of the Extrahepatic Bile Duct|Cholangiocarcinoma of the Gallbladder|Localized Unresectable Adult Primary Liver Cancer|Metastatic Extrahepatic Bile Duct Cancer|Recurrent Adult Primary Liver Cancer|Recurrent Extrahepatic Bile Duct Cancer|Unresectable Extrahepatic Bile Duct Cancer | Drug: 6,8-bis(benzylthio)octanoic acid | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1|Phase 2 | 2013-05-01 | 2017-03-13 |
| NCT01839981 | Acinar Cell Adenocarcinoma of the Pancreas|Duct Cell Adenocarcinoma of the Pancreas|Recurrent Pancreatic Cancer|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer | Drug: 6,8-bis(benzylthio)octanoic acid | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2013-07-01 | 2017-03-13 |
| NCT01835041 | Acinar Cell Adenocarcinoma of the Pancreas|Duct Cell Adenocarcinoma of the Pancreas|Recurrent Pancreatic Cancer|Stage IV Pancreatic Cancer | Drug: 6,8-bis(benzylthio)octanoic acid|Drug: oxaliplatin|Drug: leucovorin calcium|Drug: irinotecan hydrochloride|Drug: fluorouracil|Other: laboratory biomarker analysis | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1 | 2013-04-01 | 2017-03-13 |
| NCT02484391 | Granulocytic Sarcoma|Recurrent Adult Acute Myeloid Leukemia | Drug: 6,8-Bis(benzylthio)octanoic Acid|Drug: Cytarabine|Procedure: Hematopoietic Cell Transplantation|Drug: Mitoxantrone Hydrochloride | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 2 | 2015-09-01 | 2017-02-20 |
| NCT02472626 | Untreated Adult Acute Myeloid Leukemia | Drug: 6,8-Bis(benzylthio)octanoic Acid|Drug: Cytarabine|Drug: Daunorubicin Hydrochloride | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 1|Phase 2 | 2015-12-01 | 2017-01-17 |