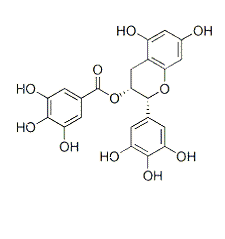
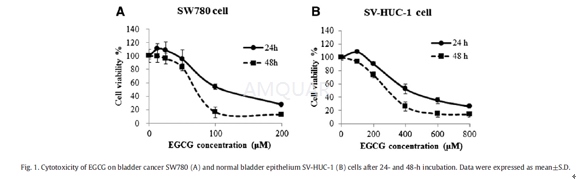
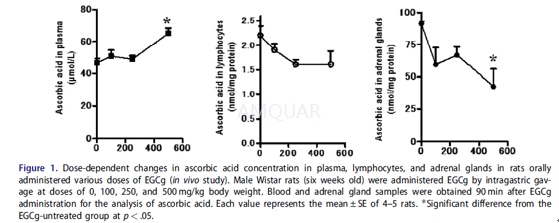
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00951834 | Alzheimer's Disease | Drug: Epigallocatechin-Gallate|Drug: Placebo | Charite University, Berlin, Germany | Phase 2|Phase 3 | 2009-10-01 | 2016-01-20 |
| NCT01357681 | Huntington Disease | Drug: (2)-epigallocatechin-3-gallate (EGCG)|Drug: Placebo | Charite University, Berlin, Germany | Phase 2 | 2011-09-01 | 2015-06-15 |
| NCT01744587 | NPC | Other: Epigallocatechin Gallate (EGCG)|Dietary Supplement: Placebo | National Health Research Institutes, Taiwan|Mackay Memorial Hospital|Taichung Veterans General Hospital|National Cheng-Kung University Hospital|Chang Gung Memorial Hospital|China Medical University Hospital|National Taiwan University Hospital | Phase 2|Phase 3 | 2013-04-01 | 2016-11-08 |
| NCT00942422 | Multiple Myeloma and Plasma Cell Neoplasm|Precancerous Condition | Dietary Supplement: defined green tea catechin extract|Genetic: gene expression analysis|Genetic: protein analysis|Other: laboratory biomarker analysis | Barbara Ann Karmanos Cancer Institute|National Cancer Institute (NCI) | Phase 2 | 2009-11-01 | 2015-03-18 |
| NCT02008721 | Multiple System Atrophy | Drug: EGCG as putative neuroprotective agent|Drug: Placebo | Dr. Johannes Levin|German Center for Neurodegenerative Diseases (DZNE)|Deutsche Parkinson Vereinigung|Deutsche Stiftung Neurologie|ParkinsonFonds Deutschland gGmbH|Ludwig-Maximilians - University of Munich | Phase 3 | 2014-01-01 | 2016-12-28 |
| NCT00459407 | Adenocarcinoma of the Prostate|Stage I Prostate Cancer|Stage II Prostate Cancer | Dietary Supplement: defined green tea catechin extract|Drug: placebo|Other: immunohistochemistry staining method|Other: immunoenzyme technique|Other: laboratory biomarker analysis|Procedure: biopsy|Other: mass spectrometry|Other: high performance liquid chromatography | National Cancer Institute (NCI) | Phase 1 | 2007-03-01 | 2014-10-07 |
| NCT00303823 | Cervical Cancer|Cervical Intraepithelial Neoplasia Grade 1|Human Papilloma Virus Infection | Drug: placebo|Dietary Supplement: defined green tea catechin extract|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2005-09-01 | 2015-04-14 |
| NCT00799890 | Multiple Sclerosis | Drug: Sunphenon EGCG|Drug: Placebo | Charite University, Berlin, Germany|TAIYO EUROPE | Phase 2|Phase 3 | 2009-05-01 | 2016-03-21 |
| NCT01511263 | Primary Amyloidosis of Light Chain Type | Drug: Diuretics (plus antiarrhythmic drugs, i.e. amiodarone, in case of complex ventricular arrhithmias)|Drug: Diuretics (plus antiarrhythmic drugs, i.e. amiodarone, in case of complex ventricular arrhythmias) plus EGCG | IRCCS Policlinico S. Matteo | Phase 2 | 2012-01-01 | 2016-02-24 |
| NCT00134381 | Healthy | Drug: Green Tea | Rutgers, The State University of New Jersey|Rutgers University | Phase 2 | 2003-05-01 | 2015-02-02 |
| NCT00573885 | Lung Cancer|Tobacco Use Disorder | Drug: defined green tea catechin extract|Other: placebo | British Columbia Cancer Agency|National Cancer Institute (NCI)|University of Cincinnati | Phase 2 | 2008-01-01 | 2012-03-07 |
| NCT00611650 | Lung Cancer|Precancerous Condition|Tobacco Use Disorder | Drug: defined green tea catechin extract|Other: placebo | British Columbia Cancer Agency|National Cancer Institute (NCI)|University of Cincinnati | Phase 2 | 2006-10-01 | 2012-03-07 |
| NCT01993966 | Urothelial Carcinoma | | National Taiwan University Hospital | | 2014-01-01 | 2013-11-19 |
| NCT00596011 | Prostatic Hyperplasia | Drug: Polyphenon E|Drug: Placebo | H. Lee Moffitt Cancer Center and Research Institute|National Cancer Institute (NCI) | Phase 2 | 2007-12-01 | 2016-11-15 |
| NCT00253643 | Precancerous Condition|Prostate Cancer | Dietary Supplement: green tea catechin extract|Dietary Supplement: fish oil|Other: placebo|Other: placebo | OHSU Knight Cancer Institute|United States Department of Defense|National Cancer Institute (NCI) | | 2005-07-01 | 2015-02-17 |
| NCT00917735 | Breast Cancer | Drug: Green tea extract supplement|Other: Placebo | University of Minnesota - Clinical and Translational Science Institute|National Cancer Institute (NCI) | Phase 2 | 2009-07-01 | 2016-01-25 |