-
生物活性
Verapamil hydrochloride is an α-adrenergic receptor antagonist and calcium channel protein inhibitor that blocks the L-type Ca2+ channels in smooth and cardiac muscle cells. Verapamil is an antiarrhythmic agent and vasodilator known to reduce the renal clearance of digoxin and induce apoptosis in primary and metastatic colon adenocarcinoma human cell lines in vitro. It has been observed that verapamil can induce currents by itself, presumably by acting on the potassium and chloride leakage. Verapamil has also been used as an inhibitor of drug efflux pump proteins such as Mdr.
-
体外研究
-
体内研究
-
激酶实验
Calcium Current Measurements[1]
The ICa was recorded using standardwhole-cell patch clamp. The myocytes were placed into the perfusion channel(volume 50μl) allowing rapid (<1 s) and complete exchange of externalsolutions. Sodium current was inhibited using -50-mV holding potential and 20μMtetrodotoxin (TTX) in the external solution, and potassium currents were blockedby replacing K+ with Cs+ inboth external and internalsolution. The standard pulse protocol for recordingcalcium currents consisted of a continuous train of 70-ms-long voltage pulseselicited every 3 s from a holding potential of -50 mV. Calcium channels were keptin phosphorylated state by including 50 μM cAMP to theinternal solution and 10μM 3-isobutyl-1-methylxanthine (IBMX; a membrane- permeantphosphodiesterase inhibitor) into the external solution. These conditions werechosen to standardize cell status by reducing the variability of calciumcurrents due to variable phosphorylation level of calcium channels in isolatedmyocytes, to reduce calcium current rundown, and to eliminate the consequencesof eventual interactions of the studied drugs with the phosphorylation/dephosphorylationpathways. Experiments were carried out at 20 to 24°C.
Solutionsfor Patch-Clamp Recordings
The external solution contained 135Mm NaCl,5.4mM CsCl, 10mM HEPES, 5mM MgCl2, 0.33 mM NaH2PO4,1 mM CaCl2, 0.02 mM TTX, and 0.01 mM IBMX, pH 7.3. This solution wasalso used as the vehicle for the drugs. The internal solution contained 135 mMCsCH3SO3, 10 mM CsCl, 10 mM HEPES, 1 mM EGTA, 3 mM MgSO4,3 mM ATP, and 0.050 mM cAMP, pH 7.3.
-
细胞实验
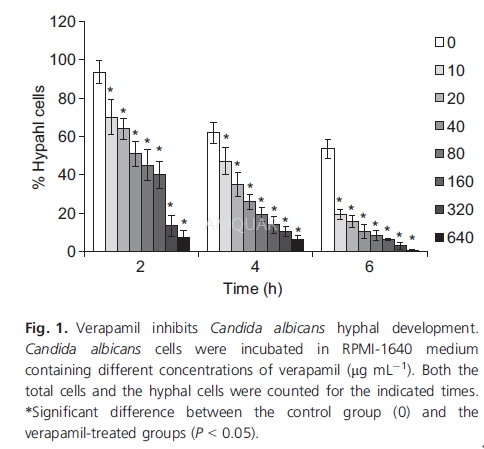
Hyphal induction[2]
Candida albicans hyphae were induced inliquid RPMI-1640 medium. Overnight cultured cells of strain SC5314 or NKF152were harvested, washed with phosphate-buffered saline (PBS), and diluted withRPMI-1640 medium to an optical absorbance at 600 nm (A600 nm) of 0.1. Verapamilwas then added at 0, 10, 20, 40, 80, 160, 320 or 640μg /mL. Thecell suspensions were cultured at 37℃ with shaking forthe indicated times. Cells were centrifuged, fixed with 4% formaldehyde andobserved with a light microscope. Both hyphal cells and total cells werecounted, and the percentage of hyphal cells was calculated as the number ofhyphal cells divided by the number of total cells. At least 10 microscopicfields were detected.
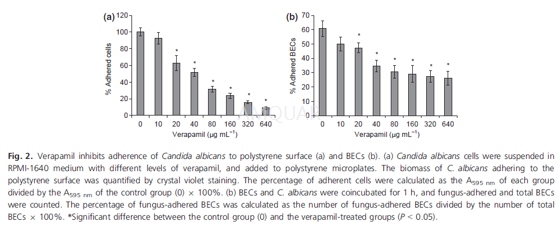
Adhesionto polystyrene
Overnight cultured cells were diluted withRPMI-1640 medium as described above, and the suspension was added into 24-wellpolystyrene microplates. Verapamil was supplemented into the wells as in hyphalinduction. The plates were incubated at 37℃ for 4 h, washedwith PBS buffer, and stained using 0.1% crystal violet for5 min. The wells werethen washed again with PBS buffer, and 500μL of 10%acetic acid was added to extract the dye. The optical absorbance at 595 nm(A595 nm) of the extract was determined using a spectrophotometer. Thepercentage of adhering cells were calculated as the A595 nm of each groupdivided by the A595 nm of the control group 9 100%. The cells adhering to the wellsurface were also examined by light microscopy.
Adhesionto buccal epithelial cells (BECs)
Adhesion to BECs was assessed. BECs werecollected from healthy individuals, washed with sterilized physiological salineand suspended in the same solution at 2.5 x 105 /mL. Cells of C.albicans strain SC5314 or NKF152 were cultured overnight in modified NGY medium(0.1% peptone, 0.1% yeast extract, 0.4% glucose), harvested, washed with salineand suspended in the same solution at 2.5 x 106 yeast cells /mL.Then, 100μL of BECs and 100 μL of yeast cells were mixed. Verapamil was added to the mixtures as describedfor hyphal induction. The mixture was incubated with shaking at 30℃ for 1 h,washed with saline, fixed with 4% formaldehyde and observed. The percentage offungus-adhered BECs was calculated as the number of fungus-adhered BECs dividedby the number of total BECs x 100%.
-
动物实验
Animal[3]
Adult male Sprague-Dawley (SD) rats (250-350g) were used in the present study.
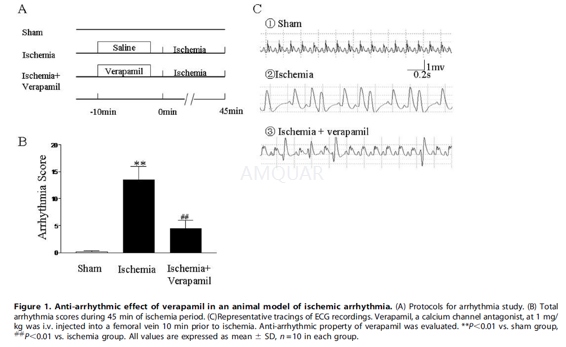
Invivo Arrhythmia Study
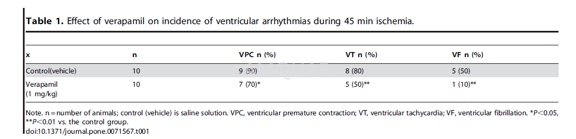
SD rats weighing 250 to 350 g wereanesthetized by i.p. injection of 3% pentobarbital (60 mg/kg). Supplementaldoses of sodium pentobarbital were given as necessary to maintain a uniformlevel of anesthesia. Arrhythmia was induced by temporary occlusion of the leftanterior descending (LAD) coronary artery from the area immediately below theleft atrial appendage to the right portion of the left ventricle with a 6–0silk suture for 45 min. Mean arterial blood pressure (MABP) was continuouslymonitored via a saline-filled catheter inserted into the right femoral artery,which was connected to a pressure transducer. PE50 catheters were inserted intothe left ventricle (LV) from the right carotid artery forthe measurement of LVpressure (LVSP) with a pressure transducer. Before and during ischemia,electrocardiogram (ECG) was used to measure heart rate (HR) and the incidenceof arrhythmias including percent of animals with premature ventricularcontractions (PVC), percent of animals with ventricular tachycardia (VT), andpercent of animals with ventricular fibrillation (VF).
Verapamil (1 mg/kg) was injected i.v. intoa femoral vein10 min prior to ischemia. A sham group underwent the same surgicalprocedures, except the suture underneath the LAD was left untied.
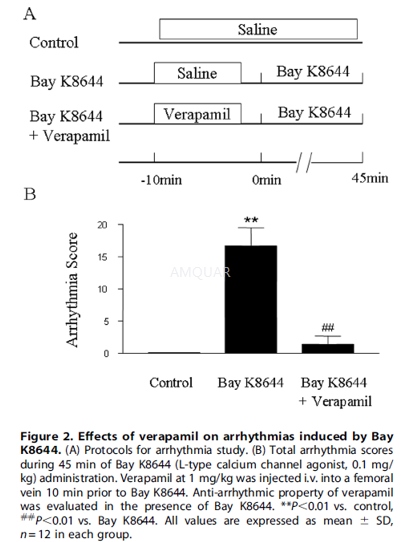
In another series of experiment, arrhythmiawas induced by Bay K8644, an L-type calcium channel agonist, at a dose of 0.1mg/kg given i.v. into the FV. Verapamil (1 mg/kg) was administered10 min priorto Bay K8644. All injections were performed within30 sec.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Zahradnik I, Minarovic I, Zahradnikova A. Inhibition of the cardiac L-type calcium channel current by antidepressant drugs. J Pharmacol Exp Ther. 2008;324(3):977-984.
[2] Yu Q, Ding X, Zhang B, et al. Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization. FEMS Yeast Res. 2014;14(4):633-641.
[3] Zhou P, Zhang SM, Wang QL, Wu Q, Chen M, Pei JM. Anti-arrhythmic effect of verapamil is accompanied by preservation of cx43 protein in rat heart. PLoS One. 2013;8(8):e71567.
[4] Bellamy, W.T. 1996. Annu. Rev. Pharmacol. Toxicol. 36: 161-183.
[5] Kantola, T., et al. 1998. Clin. Pharmacol. Ther. 64: 177-182.
[6] Verschraagen, M., et al. 1999. Pharmacol. Res. 40: 301-306.
分子式
C27H38N2O4.HCl |
分子量
491.06 |
CAS号
152-11-4 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
>90 mg/mL |
Water
45 mg/mL |
Ethanol
10 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01467687 | Healthy | Drug: verapamil controlled release | Synerx Pharma, LLC | Phase 1 | 2007-07-01 | 2011-11-08 |
| NCT01471704 | Healthy | Drug: INX-08189 50 mg|Drug: 240 mg verapamil HCL ER|Drug: 50 mg dose of INX-08189 and 240 mg verapamil HCL ER | Bristol-Myers Squibb | Phase 1|Phase 2 | 2011-10-01 | 2012-06-21 |
| NCT00647673 | Healthy | Drug: Verapamil HCL Extended-Release Capsules 300 mg|Drug: Verelan庐 PM Extended-Release Capsules 300 mg | Mylan Pharmaceuticals | Phase 1 | 2006-01-01 | 2008-03-31 |
| NCT00983242 | Pharmacokinetics | Drug: Colchicine|Drug: Verapamil HCl ER|Drug: Colchicine | Mutual Pharmaceutical Company, Inc. | Phase 1 | 2008-09-01 | 2009-10-12 |
| NCT00668967 | Healthy Volunteers | Drug: verapamil|Drug: verapamil | Pfizer | Phase 1 | 2008-02-01 | 2009-07-07 |
| NCT02454608 | Sinusitis|Nasal Polyps | Drug: Verapamil HCl|Other: Placebo | Massachusetts Eye and Ear Infirmary | | 2015-05-01 | 2016-12-28 |
| NCT01607073 | Dravet Syndrome | Drug: Verapamil | Beverly S. Wical, M.D.|Mayo Clinic|Ann & Robert H Lurie Children's Hospital of Chicago|Dartmouth-Hitchcock Medical Center|Gillette Children's Specialty Healthcare | Phase 2 | 2012-04-01 | 2015-03-24 |
| NCT02235558 | Ischemic Stroke | Drug: Verapamil | Justin Fraser|University of Kentucky | Phase 1 | 2013-02-01 | 2017-01-19 |
| NCT02111317 | Healthy Subjects|Pharmacokinetics of ASP015K | Drug: ASP015K|Drug: verapamil | Astellas Pharma Global Development, Inc.|Janssen Biotech, Inc.|Astellas Pharma Inc | Phase 1 | 2013-10-01 | 2014-04-07 |
| NCT01402427 | Coronary Disease|Verapamil Toxicity | Drug: Verapamil|Drug: Placebo | State Health Center, Hungary | | 2011-03-01 | 2014-04-30 |
| NCT02823106 | Ischemic Stroke | Drug: Verapamil and Citicoline|Other: Placebo | Justin Fraser|University of Kentucky | Phase 1 | 2016-08-01 | 2016-09-21 |
| NCT02372253 | Type 1 Diabetes Mellitus | Drug: Verapamil|Drug: Placebo | University of Alabama at Birmingham|Juvenile Diabetes Research Foundation | Phase 2 | 2015-01-01 | 2016-10-21 |
| NCT01669304 | Vestibular Migraine|Chronic Subjective Dizziness | Drug: Verapamil|Drug: Sertraline | Mayo Clinic | Phase 1 | 2012-08-01 | 2015-08-20 |
| NCT01197781 | Drug Drug Interactions|Healthy Volunteers | Drug: FOSTAMATIN|Drug: Verapamil | AstraZeneca | Phase 1 | 2010-09-01 | 2010-11-19 |
| NCT00559169 | Catamenial Epilepsy | Drug: verapamil hyrochloride | University Health Network, Toronto | | 2009-02-01 | 2010-04-07 |
| NCT02144792 | Epilepsy | Drug: [11C] -verapamil PET | Seoul National University Hospital | Phase 2 | 2013-05-01 | 2014-05-19 |
| NCT01126307 | Epilepsy|Seizures | Drug: Verapamil|Drug: placebo | University Health Network, Toronto | | 2010-06-01 | 2010-05-18 |
| NCT02912663 | Ischemic Stroke | Drug: Verapamil and Magnesium Sulfate|Other: Placebo | Justin Fraser|University of Kentucky | Phase 1 | 2017-03-01 | 2017-02-04 |
| NCT01645709 | Osteoarthritis of the Knee | Drug: Verapamil|Drug: Placebo | Calosyn Pharma, Inc.|Health Decisions | Phase 1|Phase 2 | 2012-04-01 | 2014-08-26 |
| NCT01943487 | Drug-Drug Interaction (DDI)|Healthy Subjects | Drug: EC905|Drug: verapamil | Astellas Pharma Europe B.V.|Astellas Pharma Inc | Phase 1 | 2009-08-01 | 2014-05-29 |
| NCT00707551 | Healthy | Drug: Ketoconazole|Drug: Verapamil|Drug: AZD1305 | AstraZeneca | Phase 1 | 2008-07-01 | 2010-12-02 |
| NCT02209155 | Episodic Cluster Headache | Drug: R-verapamil 75 mg tablet|Drug: Placebo | Center Laboratories, Inc. | Phase 2 | 2013-11-01 | 2015-07-31 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们