-
生物活性
Doxycycline hyclate, also called doxycycline is an antimicrobial tetracycline that acts as an inhibitor of a wide range of matrix metalloproteinases (MMPs). This product has been observed to significantly decrease the levels and activity of metalloproteinase-9 (MMP-9) in corneal erosion studies. Rheumatoid arthriitis studies indicate that doxycycline can block the degradation of type II collagen, and not type I collagen, as well as down regulate the expression levels and activity of MMP-8. Increases and promotes smooth muscle cell adhesion; inhibits proliferation and migration. Protects the microvasculature by inhibiting plasminogen systems. Additionally, doxycycline has been observed to alter the expression of apicoplast genes in plasmodium falciparum, and inhibit tissue formation. Doxycycline hyclate is an inhibitor of MMP-1 and MMP-8.
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Cell culture[1]
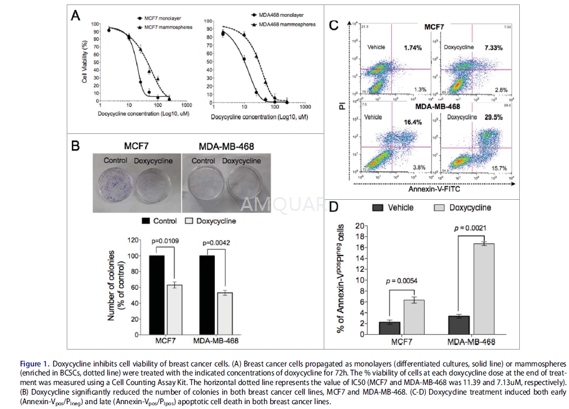
The human breast cancer cell lines MCF-7,and MDA-MB-468 were grown in RPMI-1640 containing 10% fetal bovine serum (FBS),with penicillin (100U/mL) and streptomycin (100μg/mL). Allcells were cultured in a 5% CO2 incubator at 37 oC with 5%relative humidity.
Mammospherecultures
Cells were plated in ultra-low-attachment6-well plates at a density of 1x104/mL, and cultured in serum-freeDMEM/F12 media, supplemented with B27 (1:50), 20ng/mL recombinant EGF, 20ng/mLrecombinant bFGF, 0.4% bovine serum albumin, and 4μg/mL insulin.The medium containing the growth factors were replaced every 3 d. After theformation of the spheres (day 8), the cells were collected by gentlecentrifugation for experiments. For the mammosphere forming efficiency experiments,1000 cells were plated per well on 6-well plates and treated with doxycyclineat the indicated concentrations or vehicle. 8-10 d later the number ofmammospheres per well was counted and the percent of cells forming mammosphereswas calculated.
Cellviability assay
Cells were seeded in 96-well plates at adensity of 5000 cells/well. The cells were incubated with doxycycline atconcentrations of 0, 1, 2, 10, 25, 50, 100 and 250μM for 72hours. After adding thesolution of the Cell Counting Assay Kit-8 to the wells,the cells were incubated for another 2 hours. The absorbance was measured witha microplate reader at 450 nm. The amount of the formazan dye, generated by theactivated dehydrogenases in the cells, was directly proportional to the number ofliving cells. Medium alone was used as the blank. The mammosphere proliferationassays were performed in a similar manner. % cell viability = Experimentalgroup optical density (OD) value / Control group (OD) value x100%. The drugconcentration that inhibited 50% of the growth of control cells (IC50) was calculatedby SPSS v19.0 software. All experiments were performed3 times independently.
Flowcytometry analysis
Cells were treated with doxycycline for 72hours in monolayer cultures. On day 4, the surviving cells were removed and stainedfor CD44 and CD24 expression on the cell surface, using FITC-conjugatedanti-CD44 and PE-conjugated anti-CD24 antibodies according to themanufacturer’s instructions. After incubation with antibodies for 30 min at 4oC,cells were analyzed by flow cytometry and the CD44+CD24-/low BCSC population was estimated via flow cytometry.
Cellapoptosis analysis
Cells were seeded at 1x105 cellsper well in 6-well plates and allowed to adhere overnight, then treated withdifferent concentrations of doxycycline (0μM, IC50concentrations of doxycycline) for 72 hours. After washing withphosphate-buffered saline, the cells were resuspended in 500μLof binding buffer and incubated with annexin V–FITC/PI following themanufacturer’s instructions. After incubation for 15 minutes at 4oC,the cells were analyzed using flow cytometry.
Colonyformation assay
1,000 cells from MCF-7, and MDA-MB-486 celllines were plated in 6-cm dishes and allowed to adhere overnight, then treatedwith doxycycline. 14 days later, the colonies were fixed with methanol andstained with 0.5% crystal violet. Colonies with over 50 cells were countedunder an inverted microscope. The surviving fraction was calculated accordingto the following formula: Surviving Fraction = number of colonies / number ofseeded cells x100%.
Three biologically independent experimentswere performed.

-
动物实验
Animals[2]
Male C57BL/6 mice at the age of 6–8 weeksand male Sprague Dawley rats weighting about 200 g were acclimatized for 7 daysafter arrival.
Elastaseinduced AAA mouse model
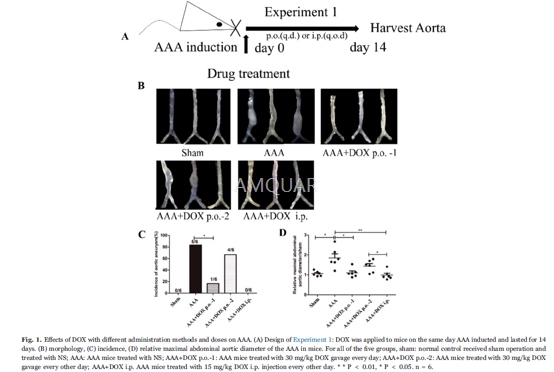
In this study, mice AAA were induced byelastase. Briefly, mice were anesthetized by chloral hydrate and placed in asupine position. The abdominal aorta of the mice was isolated and incubatedwith 1.5U pancreatic elastase for 40 min. Then the elastase was removed and theabdomen was sutured. Saline instead of elastase was used in the parallel shamoperation as AAA sham control.
Experiment1
Protectiveeffects of DOX on AAA at different doses and by different administration routes
Mice received the operation of AAAinduction were divided into five groups, and treated with DOX or saline (NS) onthe same day of AAA induction and lasted for 14 days. DOX treatment includedthree groups: gavage (p.o.) administration of 30 mg/kg of DOX every day(AAA+DOX p.o.-1) or every other day (AAA+DOX p.o.-2) and intraperitoneal(AAA+DOX i.p.) injection of 15 mg/kg of DOX every other day. The sham group(the AAA sham control) and AAA group (the AAA model) were treated with NSinstead of DOX according to the same regimen as described in the DOX treatedgroups. After treatment for 14 days, the mice were killed and severity of AAAwas evaluated.
Experiment2
Therapeuticeffects of DOX on the established AAA
AAA was induced in mice abdominal aortas byelastase according to the procedure described above and waited for 14 days todevelop AAA. The mice were then treated with 15 mg/kg DOX by i.p. injectionevery other day and 7 injections in total 14 days (AAA+DOX). NS substituted forDOX in the sham group and AAA group. After treatment for 14 days, the mice werekilled and severity of AAA was evaluated.
Harvestof mice aortas
The mice aortas in Experiments 1 and 2 wereisolated and photographed by a digital camera to measure the external maximaldiameter of the abdominal aortas. AAA incidence was defined as a ≥ 50%increase in aortic diameters compared to the sham group. The relative dilationratio was expressed as the ratio of maximal aneurysmal diameter of the AAA groupsto the sham group.
Histologicalanalysis
The abdominal aortas were dissected andfixed in 4% paraformaldehyde for 6 h and then dehydrated in 20% sucrosesolution. Specimens were embedded in Tissue-Tek optimal cutting temperature(O.C.T.) compound in liquid nitrogen, then sliced into 7 μm sections. Then thefrozen sections were stained with Hematoxylin-Eosin (HE) and aldehyde fuchsin,which was used to specifically stain the elastic fibers. Elastin degradationscores were determined according to the following standards: 1, 25%degradation; 2, 25~50% degradation; 3, 50~75% degradation; 4, greater than 75%degradation.
Immunohistochemistry
Immunohistochemical staining with MAC-2 (amacrophage marker), MMP-2 and MCP-1 antibodies was used to evaluateinflammation and elastin degradation. Briefly, the slices were incubated in 3%H2O2 for 10 min to eliminate endogenous peroxidase activity and blocked with 5%goat serum for 1 h at room temperature, and then incubated with primaryantibodies overnight at 4 °C. Subsequently, the slices were washed withphosphate buffer saline (PBS) and incubated (1 h, 37 °C) with peroxidaseconjugated goat anti-rabbit or mice second antibodies. Finally, the slices wereincubated with diaminobenzidine(DAB) to visualize peroxidase activity by lightmicroscopy.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Zhang L, Xu L, Zhang F, Vlashi E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle. 2017;16(8):737-745.
[2] Yu M, Chen C, Cao Y, Qi R. Inhibitory effects of doxycycline on the onset and progression of abdominal aortic aneurysm and its related mechanisms. Eur J Pharmacol. 2017.
[3] Wernig et al (2008) A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat.Biotechnol. 26 916.
[4] Burggraf et al (2007) Doxycycline inhibits MMPs via modulation of plasminogen activators in focal cerebral ischemia. Neurobiol.Dis. 25 506.
[5] Franco et al (2006) Doxycycline alters vascular smooth muscle cell adhesion, migration, and reorganization of fibrillar collagen matrices. Am.J.Pathol. 168 1697.
分子式
C22H25ClN2O8 |
分子量
480.9 |
CAS号
24390-14-5 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
100 mM |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01087476 | Mucositis | Drug: Doxycycline hyclate | Metropolitan Autonomous University|Instituto Nacional de Cancerologia de Mexico | Phase 2 | 2010-05-01 | 2010-03-15 |
| NCT01323101 | Cystic Fibrosis | Drug: Doxycycline | University of Southern California | Phase 4 | 2008-04-01 | 2017-03-21 |
| NCT01475708 | Lyme Borreliosis | Drug: Doxycycline | University Medical Centre Ljubljana | | 2011-05-01 | 2016-03-23 |
| NCT02341209 | Cutaneous T-cell Lymphoma|Mycosis Fungoides|Sezary Syndrome | Drug: Doxycycline monohydrate | Rochester General Hospital | Phase 2 | 2017-08-01 | 2017-03-01 |
| NCT01375491 | Type 2 Diabetes|Obesity | Drug: Doxycycline|Other: Placebo | University of California, San Diego|Ruth L. Kirschstein National Research Service Award|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)|National Center for Research Resources (NCRR) | Phase 4 | 2009-10-01 | 2013-05-17 |
| NCT00635609 | Acne Vulgaris | Drug: Doxycycline hyclate (Doryx)|Drug: Doxycycline hyclate | Warner Chilcott | Phase 4 | 2008-03-01 | 2012-04-18 |
| NCT02929121 | Lymphedema|Lymphatic Filariasis|Filariasis | Drug: Doxycycline|Drug: Placebo | The Task Force for Global Health|United States Agency for International Development (USAID) | Phase 3 | 2017-07-01 | 2017-03-13 |
| NCT02929134 | Lymphedema|Lymphatic Filariasis|Filariasis | Drug: Doxycycline|Drug: Placebo | The Task Force for Global Health|United States Agency for International Development (USAID) | Phase 3 | 2017-07-01 | 2017-03-13 |
| NCT02927496 | Lymphedema|Lymphatic Filariasis|Filariasis | Drug: Doxycycline|Drug: Placebo | The Task Force for Global Health|United States Agency for International Development (USAID) | Phase 3 | 2017-07-01 | 2017-03-13 |
| NCT02774993 | Tuberculosis | Drug: Doxycycline|Drug: placebo | National University Hospital, Singapore|Tan Tock Seng Hospital|National University, Singapore|A*Star | Phase 2 | 2015-09-01 | 2016-05-12 |
| NCT02207556 | Primary Systemic Amyloidosis | Drug: Doxycycline | Medical College of Wisconsin | Phase 2 | 2014-10-01 | 2016-08-18 |
| NCT00688064 | Severe Acne Vulgaris | Drug: Adapalene BPO Gel associated with Doxycyline Hyclate|Drug: Vehicle Gel associated with Doxycycline Hyclate | Galderma | Phase 3 | 2008-08-01 | 2010-03-31 |
| NCT02775695 | Resectable Pancreatic Cancer | Drug: Doxycycline | Medical College of Wisconsin | Phase 2 | 2016-07-01 | 2016-05-13 |
| NCT02850913 | Seizures | Drug: Doxycycline|Other: Placebo | Makerere University|University of Oxford | Phase 2 | 2016-09-05 | 2017-02-22 |
| NCT01935622 | Non-ischemic Cardiomyopathy|Systolic Heart Failure (NYHA II-III) | Drug: Doxycycline|Drug: placebo | Virginia Commonwealth University | Phase 2 | 2012-07-01 | 2014-08-19 |
| NCT01872715 | Papulopustular Rosacea | Drug: Oracea | Galderma Laboratories, L.P. | Phase 4 | 2013-03-01 | 2016-01-20 |
| NCT00547170 | Endometritis | Drug: Doxycycline pre-operatively|Drug: Doxycycline post-operatively | University of Pittsburgh|Tu Du Hospital | Phase 4 | 2007-01-01 | 2008-05-14 |
| NCT02562651 | Vascular Diseases|Cardiovascular Diseases|Acute Myocardial Infarction | Drug: Doxycycline|Other: Standard care for STEMI | Russian Academy of Medical Sciences | Phase 2|Phase 3 | 2014-02-01 | 2016-12-10 |
| NCT00066027 | Periodontitis | Drug: 20 mg doxycycline hyclate|Drug: Placebo | University of Nebraska|National Institute of Dental and Craniofacial Research (NIDCR) | Phase 3 | 2002-06-01 | 2015-12-01 |
| NCT01469585 | Breakthrough Bleeding | Drug: Doxycycline | University of Hawaii|Charles Drew University of Medicine and Science|Meharry Medical College | | 2011-11-01 | 2014-08-18 |
| NCT01727973 | Graves Ophthalmopathy|Graves Disease|Eye Diseases|Thyroid Diseases|Endocrine System Diseases|Eye Diseases, Hereditary|Hyperthyroidism|Autoimmune Diseases|Immune System Diseases | Drug: Doxycycline | Sun Yat-sen University | Phase 1|Phase 2 | 2012-10-01 | 2013-12-07 |
| NCT01112059 | Cystic Fibrosis | Drug: Doxycycline|Other: placebo | University of Alabama at Birmingham|Cystic Fibrosis Foundation Therapeutics | | 2008-11-01 | 2017-01-11 |
| NCT01847976 | Pain | Drug: Doxycycline | Ottawa Hospital Research Institute|Canadian Breast Cancer Foundation | Phase 2 | 2013-08-01 | 2016-11-17 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们