-
生物活性
Tazemetostat (EPZ-6438) an EHZ2 inhibitorin clinical development, has shown activity in both preclinical models of canceras well as in patients with lymphoma or INI1-deficient solid tumors. EPZ-6438 is a potent, selective, and orally bioavailable small-molecule inhibitor of EZH2 enzymatic activity with Ki value of 2.5±0.5 nM. EPZ-6438 inhibited the activity of human PRC2-containing wild-type EZH2 with an inhibition constant (Ki) value of 2.5 ± 0.5 nM, and similar potency was observed for EZH2 proteins bearing all known lymphoma change-of-function mutations. EPZ-6438 displayed a 35-fold selectivity versus EZH1 and >4,500-fold selectivity relative to 14 other HMTs tested. In vitro treatment of SMARCB1-deleted MRT cell lines with EPZ-6438 induced strong antiproliferative effects with IC50 values in the nanomolar range, whereas the control (wild-type) cell lines were minimally affected. EPZ-6438 was well tolerated at all doses with minimal effect on body weight.
-
体外研究
-
体内研究
0.5% NaCMC 加 0.1% Tween 80 水溶液
-
激酶实验
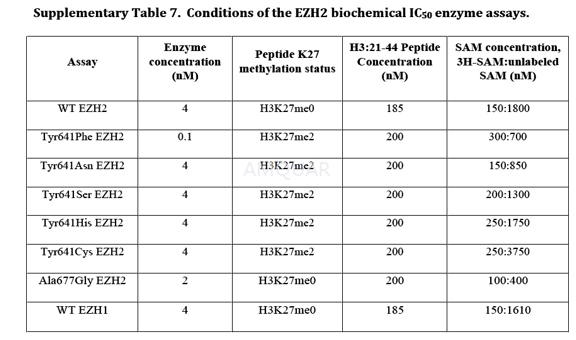
Determination of Enzyme Inhibition IC50 Values[1]
10-point curves of EPZ-6438 were made usingserial 3-fold dilutions in DMSO, beginning at 2.5mM (final top concentration ofcompound was 50μM and the DMSO was2%). A 1μL aliquot the inhibitor dilutionseries was spotted in a 384-well microtiter plate. The 100% inhibition controlconsisted of 1 mM final concentration of the product inhibitor S-adenosylhomocysteine,(SAH). Compound was incubated for 30 min with 40μL per well of 5 nM PRC2 (finalassay concentration in 50μL was 4 nM) in 1X assay buffer (20 mM Bicine [pH7.6], 0.002% Tween 20, 0.005% Bovine Skin Gelatin and 0.5 mM DTT). 10μL perwell of substrate mix comprising assay buffer 3H-SAM, unlabeled SAM,and peptide representing histone H3 residues 21-44 containing C-terminal biotin(appended to a C-terminal amide-capped lysine) were added to initiate thereaction (both substrates were present in the final reaction mixture at theirrespective Km values, an assay format referred to as "balancedconditions". The final concentrations of substrates and methylation stateof the substrate peptide are indicated for each enzyme in Supplementary Table 7.Reactions were incubated for 90 min at room temperature and quenched with 10μLper well of 600μM unlabeled SAM, then transferred to a 384-well Flashplate and Washedafter 30 min.
-
细胞实验
Cell culture[2]
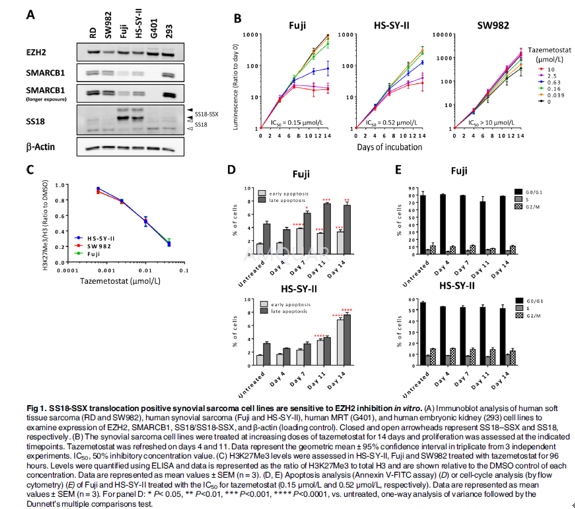
Human synovial sarcoma line HS-SY-II, Fujiand Human soft tissue sarcoma cell line SW982 (HTB-93) were cultured in RPMI +10% FBS. For examination of changes in H3K27Me3 and gene expression, each ofthe cell lines were plated to ensure cell densities were within linear log phasegrowth until sample collection. Cells were treated with either DMSO ortazemetostat as indicated. After the treatment for 96 hours (H3K27Me3 analysis)or at each time point (gene expression analysis), cells were washed with PBS.For analysis of H3K27Me3 alterations, cells were harvested and subjected tohistone extraction. For analysis of gene expression alterations, cells werelysed using a Cells-to-Ct kit according to the manufacturer's protocol.
Immunoblot
Protein concentrations were determined byBCA Protein assay. A sample solution was prepared by mixing 2 × loading buffer(β-ME Sample Treatment for Tris SDS) and water with cell lysates or extracted histones,and incubated for 5 minutes at 95°C. Immunoblot analysis was performed asfollows. The sample solutions were separated on 15–25% (for histones) or 4–20%(for other proteins) gradient agarose gel under reducing conditions andtransferred to nitrocellulose membranes and probed with the followingantibodies: rabbit polyclonal anti-EZH2 antibody, rabbit monoclonalanti-SMARCB1 antibody, mouse monoclonal anti-SS18 antibody, mouse monoclonalanti-β-actin antibody, rabbit monoclonal anti-H3K27Me3 antibody, rabbitmonoclonal anti-H3K27Me2 antibody, and rabbit polyclonal anti-total H3 antibody.Immunoblotting was performed on an iBind Western Device according to themanufacturer's instructions using horseradish peroxidase conjugated anti-rabbitIgG or anti-mouse IgG antibodies. Blots were developed with Immobilon Westernchemiluminescent HRP substrate. Immunoreactive bands were visualized by chemiluminescencewith Luminescent Image Analyzer LAS-3000 and the signals of protein bands werequantified using Multi Gauge version 3.0 software.
Proliferationassays
The cell lines were harvested with 0.25%trypsin solution, counted, diluted and dispensed at100 μL/well in collagen type1-treated 96-well plates for Fuji cells or tissue culture treated 96-wellplates for HS-SY-II and SW982 cells. The cells were also dispensed at 2 mL/wellin collagen type 1-treated 6-well plates (IWAKI) for Fuji cells or tissueculture treated 6-well plates for HS-SY-II and SW982 cells. Each of the celllines were plated to ensure cell densities were within linear log phase growthuntil measuring cell viability or the cells were replated (500 cells in 96-welland 15,000 cells in 6-well; HS-SY-II, 800 cells in 96-well and 24,000 cells in6-well; SW982, 250 cells in 96-well and 7500 cells in 6-well). The cells wereincubated under 37°C, 5% CO2 condition (day 0). Several hours later,100 μL or 2 mL of culture medium containing tazemetostat or 0.2% of DMSO ascontrol was added to each well of 96-well or 6-well plates to yield final concentrationsof 0 (control), 0.039, 0.16, 0.63, 2.5, and 10 μmol/L with final concentrationof DMSO at 0.1% for measurement of cell viability on days 4 and 7. These96-well plates were used for measuring cell viability on day 0, 4, and 7. Onday 7, the cells in the 6-well plates were harvested with 0.25% trypsinsolution and counted. The cells were replated in 96-well plates at the samedensity of cells in 96-well plates on day 0, and 100 μL of tazemetostat or DMSOcontrol was added in the plates as conducted on day 0 for measurement of cellviability on days 11 and 14. Compound/media was changed with new one on days 4and 11 at concentrations of 0 (control), 0.039, 0.16, 0.63, 2.5, and10 μmol/L.On days 0, 4, 7, 11, and 14, cell viability was determined by measuring ATPcontents by CellTiter-Glo1 Luminescent Cell Viability Assay with EnVision 2102Multilabel Reader. The ratios of the measured values on days 4 and 7 to that ofday 0, and days 11 and 14 to that of replated day 7 were used. Overall plot ofproliferation of day 11 or day 14 was calculated (ratio of day 7) × (ratio ofday 11 or ratio of day 14). Three independent experiments were performed intriplicate. The mean 50% inhibitory concentration (IC50) value and 95%confidence interval (CI) were calculated based on the IC50 values generated fromseparate curves representing the growth activity versus tazemetostatconcentration of 3 independent experiments. Statistical analyses were performedusing the GraphPad Prism version 6.02.
Cellcycle and apoptosis assays
Fuji and HS-SY-II cells were incubated withtazemetostat at concentration of each cell line’s IC50, 0.15 μmol/L and 0.52μmol/L, respectively, for 4, 7, 11, and 14 days. Cell cycle analysis wasperformed after labeling DNA with propidium iodide. Apoptosis assay wasperformed using a FITC Annexin V Apoptosis Detection Kit I and Caspase-Glo1 3/7Assay according to the manufacturer's protocol. All FACS analysis was performedon an LSR Fortessa flow cytometer. Caspase 3/7 activity was determined with EnVision2102 Multilabel Reader.
-
动物实验
Mouse xenografts[3]
BIN67 (1 × 107 cells per mouse)or SCCOHT-1 cells (4 × 106 cells per mouse) were injected with a 1:1mix of Matrigel in a final volume of 200μl subcutaneously into the backs of NRG(NOD.Rag1KO.IL2RγcKO) mice. Mice were randomized to treatment arms once theaverage tumour volume reached 100mm3. EPZ-6438 was formulated in0.5% NaCMC containing 0.1% Tween-80 and 20% Captisol (pH 4.5), respectively. Inthe BIN67 xenograft model, EPZ-6438 was administered orally twice daily (BID, 0800/1600h) for 8 days at 100 or 200 mg/kg, halted for 6 days, and then was resumed atonce daily (QD) dosing for an additional 3 weeks or until humane endpoints werereached (i.e. tumour volume ∼800mm3).In the SCCOHT-1 xenograft model, EPZ-6438 was orally administered at 200 mg/kg oncedaily for 3 weeks or until humane endpoints were reached. Tumour volume andmouse weight were measured thrice weekly. Tumour volume was calculated as length× (width)2 × 0.52.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Kuntz KW, Campbell JE, Keilhack H, et al. The Importance of Being Me: Magic Methyls, Methyltransferase Inhibitors, and the Discovery of Tazemetostat. J Med Chem. 2016;59(4):1556-1564.
[2] Kawano S, Grassian AR, Tsuda M, et al. Preclinical Evidence of Anti-Tumor Activity Induced by EZH2 Inhibition in Human Models of Synovial Sarcoma. PLoS One. 2016;11(7):e0158888.
[3] Wang Y CS, Karnezis AN, Colborne S, Santos ND, Lang JD, Hendricks WP, Orlando KA, Yap D, Kommoss F, Bally MB, Morin GB, Trent JM, Weissman BE, Huntsman DG. The histone methyltransferase EZH2 is a therapeutic target in small cell carcinoma of the ovary, hypercalcaemic type. J Pathol. 2017;242(3):371-383.
分子式
C34H44N4O4 |
分子量
572.74 |
CAS号
1403254-99-8 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
5 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01897571 | B-cell Lymphomas (Phase 1)|Advanced Solid Tumors (Phase 1)|Diffuse Large B-cell Lymphoma (Phase 2)|Follicular Lymphoma (Phase 2)|Transformed Follicular Lymphoma|Primary Mediastinal Large B-Cell Lymphoma | Drug: EPZ-6438 | Epizyme, Inc. | Phase 1|Phase 2 | 2013-06-01 | 2017-01-10 |
| NCT03028103 | Diffuse Large B Cell Lymphoma|Primary Mediastinal Lymphoma|Mantle Cell Lymphoma | Drug: Tazemetostat|Drug: Fluconazole|Drug: Omeprazole|Drug: Repaglinide | Epizyme, Inc. | Phase 1 | 2017-01-01 | 2017-01-18 |
| NCT02889523 | Lymphoma, Large B-Cell, Diffuse | Drug: Tazemetostat|Drug: Rituximab|Drug: Cyclophosphamide|Drug: Vincristine|Drug: Doxorubicin|Drug: Prednisolone | The Lymphoma Academic Research Organisation|Epizyme, Inc. | Phase 1|Phase 2 | 2016-10-01 | 2016-11-16 |
| NCT03010982 | Diffuse Large B Cell Lymphoma|Primary Mediastinal Lymphoma|Mantle-Cell Lymphoma|Follicular Lymphoma|Marginal Zone Lymphoma | Drug: Tazemetostat and [14C] Tazemetostat | Epizyme, Inc. | Phase 1 | 2017-01-01 | 2017-01-03 |
| NCT02601950 | Malignant Rhabdoid Tumors (MRT)|Rhabdoid Tumors of the Kidney (RTK)|Atypical Teratoid Rhabdoid Tumors (ATRT)|Selected Tumors With Rhabdoid Features|Synovial Sarcoma|INI1-negative Tumors|Malignant Rhabdoid Tumor of Ovary|Renal Medullary Carcinoma|Epithelioid Sarcoma|Any Solid Tumor With an EZH2 GOF Mutation | Drug: Tazemetostat | Epizyme, Inc. | Phase 2 | 2015-12-01 | 2017-01-17 |
| NCT02875548 | Diffuse Large B-cell Lymphoma|Follicular Lymphoma|Malignant Rhabdoid Tumors (MRT)|Rhabdoid Tumors of the Kidney (RTK)|Atypical Teratoid Rhabdoid Tumors (ATRT)|Synovial Sarcoma|Epitheliod Sarcoma|Mesothelioma|Advanced Solid Tumors | Drug: Tazemetostat | Epizyme, Inc. | Phase 2 | 2016-08-01 | 2016-09-13 |
| NCT02860286 | Mesothelioma|BAP1 Loss of Function | Drug: Tazemetostat | Epizyme, Inc. | Phase 2 | 2016-07-01 | 2017-03-23 |
| NCT02601937 | Rhabdoid Tumors|INI1-negative Tumors|Synovial Sarcoma|Malignant Rhabdoid Tumor of Ovary | Drug: Tazemetostat | Epizyme, Inc. | Phase 1 | 2015-12-01 | 2017-03-15 |
| NCT02220842 | Lymphoma | Drug: Atezolizumab|Drug: Obinutuzumab|Drug: Tazemetostat | Hoffmann-La Roche | Phase 1 | 2014-12-18 | 2017-03-17 |
| NCT03009344 | Relapsed or Refractory B-cell Non-Hodgkin's Lymphoma | Drug: tazemetostat | Eisai Co., Ltd.|Eisai Inc. | Phase 1 | 2017-01-01 | 2016-12-30 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们




















