-
生物活性
AR-42 is a potent inhibitor of HDAC (IC50 = 16 nM in vitro). AR-42 decreases the viability of prostate cancer cell lines (IC50 = 0.40 μM). Increases the expression of p21 and the acetylation of histone H3 while decreasing the phosphorylation of Akt and the expression of Bcl-XL. AR-42 strongly suppresses the growth of PC-3 tumor xenografts, reducing levels of phospho-Akt and Bcl-XL protein in tumors.
-
体外研究
-
体内研究
-
激酶实验
In Vitro HDAC Assay[1]
HDAC activity was analyzed by using a HDACassay kit, following the manufacturer’s instruction with slight modifications.This assay was based on the ability of DU-145 nuclear extract, which is rich inHDAC activity, to mediate the deacetylation of the biotinylated [3H]-acetylhistone H4 peptide that was bound to streptavidin agarose beads. The release of[3H]-acetate into the supernatant was measured to calculate the HDACactivity. Sodium butyrate (0.25-1 mM) was used as a positive control.
-
细胞实验
Reagents and Cell Lines[2]
AR-42 was and dissolved in DMSO for the invitro studies. The canine prostate cancer cell line Ace-1 cells and prostatecarcinoma cell line LuMa were maintained in DMEM/F12 containing 10% FBS and 1%Penicillin/Streptomycin (100 unit/ml Penicillin and 100mg/ml Streptomycin) at37°C, 5% CO2, and 100% humidity.
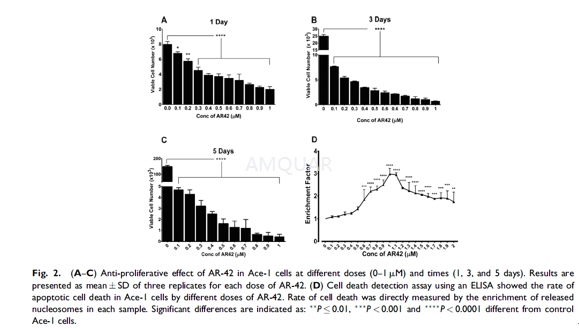
CellProliferation Assay
Ace-1 cells were seeded at 4x103 cells per well in 6-well plates (four replicates for each day). After 24hr (thepre-incubation period), cells were treated with different concentrations ofAR-42 (0–1μM) for 5 days and the medium was changed every other day. Cells wereharvested at days 1, 3, and 5, and counted using a Cellometer Auto T4 cellcounter. All wells were washed twice with Dulbecco’s Phosphate Buffered Saline(DPBS) to eliminate any detached cells. The adherent cells were trypsinized,centrifuged, and diluted 1:1 with trypan blue. Viable and dead cells werecounted and cell size measured by the Cellometer was recorded.
CellDeath Detection by ELISA
Apoptosis was measured using the Cell DeathDetection ELISA Plus Kit. Relative amounts of both monoand oligo-nucleosomesproduced from the Ace-1 cells during apoptosis were estimated using monoclonalantibodies to DNA and histones by ELISA. The cytoplasmic fractions from cellstreated with different concentrations of AR-42 (0–2 μM) for 24 hrwere transferred to a streptavidin-coated 96-well plate and incubated for 2 hrat room temperature with a mixture consisting of peroxidase-conjugated anti-DNAand biotin-labeled anti-histone. The plate was washed three times with DPBS,incubated with 2,20-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)diammonium salt and absorbance (A) was measured at 405 and 490nm with an Elisaplate reader. The enrichment factor was calculated using the following formula:Enrichment factor=mU of the sample (dying/dead cells)/mU of the correspondingnegative control where mU=absorbance (10-3).
InSitu Cell Death Detection Assay
Programmed cell death in Ace-1 cells wasdetected by labeling the DNA strand breaks in apoptotic cells using the In SituCell Death Detection Kit, Fluorescein. Ace-1 cells (6x104) were seededin each well of 8-chamber slides. AR-42 (5 μM) was addedto each well for 1–48 hr in duplicate. The experiment was repeated twice. Thecells in each well were fixed with 4% paraformaldehyde dissolved in PBS (pH7.4) for 1 hr at room temperature, washed four times with PBS, andpermeabilized with 0.1% sodium citrate for 2 min on ice. Staining was performedby incubating the cells with TUNEL reaction mixture for 60 min at 37°C in thedark and then washed three times with PBS. Cells were imaged and photographedusing a Nikon Diaphot 300 fluorescent microscope and Tucsen camera.
-
动物实验
Reagents[3]
For in vivo experiments, AR-42 was preparedas a suspension in a vehicle [10% DMSO, 0.5% methylcellulose (wt/vol) and 0.1%Tween 80 (vol/vol) in sterile water] for oral administration toxenograft-bearing athymic nude mice.
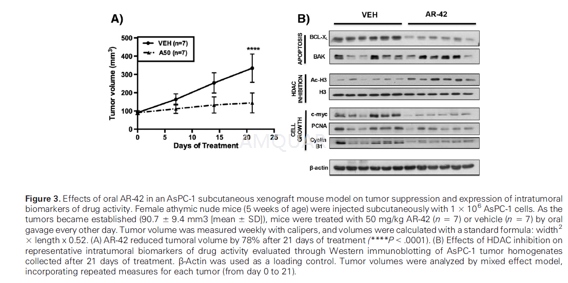
AsPC-1Xenograft Tumor Model
Each female athymic nude mouse (5 weeks ofage) received a subcutaneous injection of 1 × 106 AsPC-1 cells in atotal volume of 0.1 ml of serum-free medium containing 50% Matrigel. As thetumors became established (90.7 ± 9.4 mm3 [mean, SD]), the mice wererandomly divided into two groups (n = 7) that received the following treatmentsby oral gavage every other day: vehicle (0.5% methylcellulose/0.1% Tween 80/10%DMSO in water) and AR-42 at 50 mg/kg of body weight. Tumors were measuredweekly with calipers and their volumes were calculated with a standard formula:width2 × length × 0.52. Body weights were measured weekly. Attermination, tumors were harvested, snap-frozen in liquid nitrogen, and storedat -80°C until biomarker analysis by Western blotting.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Lu Q WD, Chen CS, Hu YD, Chen CS. Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. J Med Chem. 2005;48(17):5530-5535.
[2] Elshafae SM, Kohart NA, Altstadt LA, Dirksen WP, Rosol TJ. The Effect of a Histone Deacetylase Inhibitor (AR-42) on Canine Prostate Cancer Growth and Metastasis. Prostate. 2017;77(7):776-793.
[3] Henderson SE, Ding LY, Mo X, et al. Suppression of Tumor Growth and Muscle Wasting in a Transgenic Mouse Model of Pancreatic Cancer by the Novel Histone Deacetylase Inhibitor AR-42. Neoplasia. 2016;18(12):765-774.
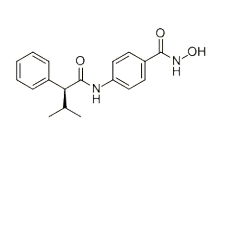
分子式
C18H20N2O3 |
分子量
312.36 |
CAS号
935881-37-1 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
40 mg/mL |
Water
<1 mg/mL |
Ethanol
40 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02282917 | Vestibular Schwannoma|Meningioma|Acoustic Neuroma|Neurofibromatosis Type 2 | Drug: AR-42 | Massachusetts Eye and Ear Infirmary|Johns Hopkins University|Mayo Clinic|Stanford University|Ohio State University|Nationwide Children's Hospital | Early Phase 1 | 2015-09-01 | 2017-02-01 |
| NCT02795819 | Renal Cell Carcinoma|Soft Tissue Sarcoma|Metastatic Disease | Drug: AR-42|Drug: Pazopanib | Virginia Commonwealth University|Arno Therapeutics|National Cancer Institute (NCI) | Phase 1 | 2016-07-08 | 2017-03-03 |
| NCT01798901 | Adult Acute Myeloid Leukemia With 11q23 (MLL) Abnormalities|Adult Acute Myeloid Leukemia With Del(5q)|Adult Acute Myeloid Leukemia With Inv(16)(p13;q22)|Adult Acute Myeloid Leukemia With t(15;17)(q22;q12)|Adult Acute Myeloid Leukemia With t(16;16)(p13;q22)|Adult Acute Myeloid Leukemia With t(8;21)(q22;q22)|Recurrent Adult Acute Myeloid Leukemia|Recurrent Childhood Acute Myeloid Leukemia|Secondary Acute Myeloid Leukemia|Untreated Adult Acute Myeloid Leukemia | Drug: HDAC inhibitor AR-42|Drug: decitabine|Other: laboratory biomarker analysis|Other: pharmacological study | Alison Walker|Ohio State University Comprehensive Cancer Center | Phase 1 | 2013-09-17 | 2017-01-19 |
| NCT02569320 | Recurrent Plasma Cell Myeloma | Drug: Dexamethasone|Drug: HDAC Inhibitor AR-42|Other: Laboratory Biomarker Analysis|Drug: Pomalidomide | Yvonne Efebera|Celgene|Ohio State University Comprehensive Cancer Center | Phase 1 | 2016-05-01 | 2016-11-22 |
| NCT01129193 | Adult Nasal Type Extranodal NK/T-cell Lymphoma|Anaplastic Large Cell Lymphoma|Angioimmunoblastic T-cell Lymphoma|Cutaneous B-cell Non-Hodgkin Lymphoma|Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Hepatosplenic T-cell Lymphoma|Intraocular Lymphoma|Nodal Marginal Zone B-cell Lymphoma|Peripheral T-cell Lymphoma|Post-transplant Lymphoproliferative Disorder|Prolymphocytic Leukemia|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Grade III Lymphomatoid Granulomatosis|Recurrent Adult Hodgkin Lymphoma|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Adult T-cell Leukemia/Lymphoma|Recurrent Cutaneous T-cell Non-Hodgkin Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Mantle Cell Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Mycosis Fungoides/Sezary Syndrome|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Refractory Multiple Myeloma|Stage III Adult Burkitt Lymphoma|Stage III Adult Diffuse Large Cell Lymphoma|Stage III Adult Diffuse Mixed Cell Lymphoma|Stage III Adult Diffuse Small Cleaved Cell Lymphoma|Stage III Adult Hodgkin Lymphoma|Stage III Adult Immunoblastic Large Cell Lymphoma|Stage III Adult Lymphoblastic Lymphoma|Stage III Adult T-cell Leukemia/Lymphoma|Stage III Chronic Lymphocytic Leukemia|Stage III Cutaneous T-cell Non-Hodgkin Lymphoma|Stage III Grade 1 Follicular Lymphoma|Stage III Grade 2 Follicular Lymphoma|Stage III Grade 3 Follicular Lymphoma|Stage III Mantle Cell Lymphoma|Stage III Marginal Zone Lymphoma|Stage III Multiple Myeloma|Stage III Mycosis Fungoides/Sezary Syndrome|Stage III Small Lymphocytic Lymphoma|Stage IV Adult Burkitt Lymphoma|Stage IV Adult Diffuse Large Cell Lymphoma|Stage IV Adult Diffuse Mixed Cell Lymphoma|Stage IV Adult Diffuse Small Cleaved Cell Lymphoma|Stage IV Adult Hodgkin Lymphoma|Stage IV Adult Immunoblastic Large Cell Lymphoma|Stage IV Adult Lymphoblastic Lymphoma|Stage IV Adult T-cell Leukemia/Lymphoma|Stage IV Chronic Lymphocytic Leukemia|Stage IV Cutaneous T-cell Non-Hodgkin Lymphoma|Stage IV Grade 1 Follicular Lymphoma|Stage IV Grade 2 Follicular Lymphoma|Stage IV Grade 3 Follicular Lymphoma|Stage IV Mantle Cell Lymphoma|Stage IV Marginal Zone Lymphoma|Stage IV Mycosis Fungoides/Sezary Syndrome|Stage IV Small Lymphocytic Lymphoma|Testicular Lymphoma|Waldenstrom Macroglobulinemia | Other: Pharmacodynamic Studies|Other: Fatigue Inventory|Other: Pharmacogenomic studies|Drug: AR-42 | Craig Hofmeister|National Cancer Institute (NCI)|Arno Therapeutics|Ohio State University Comprehensive Cancer Center | Phase 1 | 2010-05-04 | 2017-02-21 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们