| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00176488 | Breast Cancer | Drug: epirubicin|Drug: vinorelbine | University of Medicine and Dentistry of New Jersey|National Cancer Institute (NCI)|Rutgers, The State University of New Jersey | Phase 2 | 2003-06-01 | 2017-02-20 |
| NCT00496795 | Stage III Breast Cancer AJCC V7 | Other: epirubicin/docetaxel sequential | University of Bergen|Haukeland University Hospital | Phase 2 | 2007-09-01 | 2016-05-18 |
| NCT01624441 | Estrogen Receptor Negative|HER2/Neu Negative|Male Breast Carcinoma|Progesterone Receptor Negative|Recurrent Breast Carcinoma|Stage IV Breast Cancer|Triple-Negative Breast Carcinoma | Drug: Dinaciclib|Drug: Epirubicin Hydrochloride|Other: Laboratory Biomarker Analysis | National Cancer Institute (NCI) | Phase 1 | 2012-08-01 | 2017-01-31 |
| NCT00375999 | Stomach Neoplasms | Drug: Docetaxel and epirubicin | Yonsei University | Phase 2 | 2006-09-01 | 2014-01-14 |
| NCT01479036 | Breast Cancer | Drug: docetaxel and epirubicin|Drug: docetaxel and epirubicin plus endostatin | Xijing Hospital | Phase 3 | 2011-10-01 | 2011-11-21 |
| NCT01740271 | Breast Neoplasms | Drug: Epirubicin | AHS Cancer Control Alberta | Phase 2 | 2012-12-01 | 2014-10-01 |
| NCT02214602 | Carcinoma of Urinary Bladder, Superficial | Drug: Epirubicin | Mansoura University | Phase 4 | 2014-07-01 | 2016-05-18 |
| NCT00140075 | Adenocarcinoma | Drug: Epirubicin with Cyclophosphamide, followed by a Taxane|Drug: Epirubicin with a Taxane | Pfizer | Phase 3 | 2000-11-01 | 2011-05-24 |
| NCT00689156 | Breast Neoplasms | Drug: Epirubicin, cyclophosphamide and docetaxel|Drug: docetaxel, cyclophosphamide | Danish Breast Cancer Cooperative Group|Fonden Til Fremme af Klinisk- Eksperimentel Cancerforskning|Sanofi|Dako | Phase 3 | 2008-06-01 | 2013-01-03 |
| NCT01964027 | Stage IV Gastric Cancer With Metastasis | Drug: second line chemoregime for advanced gastric cancer | Anhui Medical University | Phase 2 | 2013-10-01 | 2013-10-14 |
| NCT00544232 | Breast Cancer | Drug: Epirubicin, paclitaxel, cyclophosphamide, Methotrexate, 5 FU, darbepoetin alfa|Drug: Epirubicin, Cyclophosphamide, Paclitaxel, dabepoetin alfa | German Breast Group|Pharmacia|Amgen|Bristol-Myers Squibb | Phase 3 | 2002-08-01 | 2016-02-12 |
| NCT00193024 | Breast Cancer | Drug: Docetaxel|Drug: Epirubicin | SCRI Development Innovations, LLC|Aventis Pharmaceuticals|Pharmacia and Upjohn | Phase 2 | 2001-09-01 | 2011-05-02 |
| NCT00198172 | Germ Cell Tumor | Drug: Cisplatin plus Epirubicin | Indiana University School of Medicine|Indiana University | Phase 2 | 2000-10-01 | 2014-05-30 |
| NCT00753207 | Breast Cancer | Drug: epirubicin hydrochloride|Drug: lapatinib ditosylate|Other: biomarker analysis|Other: immunohistochemistry staining method|Other: liquid chromatography|Other: mass spectrometry | Cancer Trials Ireland | Phase 1 | 2007-10-01 | 2016-02-12 |
| NCT03022123 | Diffuse Large B-cell Lymphoma | Drug: PL-doxorubicin and epirubicin | Hebei Medical University Fourth Hospital | | 2016-11-01 | 2017-01-13 |
| NCT00617370 | Breast Cancer|Adenocarcinoma of the Breast | Drug: epirubicin, cyclophosphamide, Paclitaxel, | Memorial Sloan Kettering Cancer Center|Amgen|Pfizer | | 2004-11-01 | 2015-12-21 |
| NCT01061359 | Breast Neoplasm | Drug: Epirubicin: Observational Study | Pfizer | | 1999-01-01 | 2010-12-16 |
| NCT00218205 | Prostate Cancer | Drug: Epirubicin|Drug: Estramustine Phosphate|Drug: Celecoxib | Department of Veterans Affairs, New Jersey|Pfizer | Phase 2 | 2002-07-01 | 2005-09-20 |
| NCT00705315 | Breast Cancer | Drug: Docetaxel|Drug: Epirubicin|Drug: Bevacizumab | Hellenic Oncology Research Group | Phase 1|Phase 2 | 2008-05-01 | 2010-08-18 |
| NCT00246103 | Neoplasms, Advanced | Drug: Valproic acid|Drug: Epirubicin|Drug: 5-fluorouracil|Drug: Cyclophosphamide. | H. Lee Moffitt Cancer Center and Research Institute|National Cancer Institute (NCI)|Pfizer | Phase 1 | 2004-03-01 | 2017-02-20 |





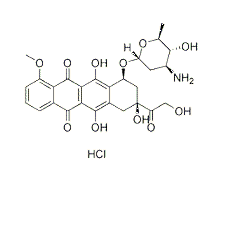
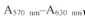 wastaken as the index of cell viability. The reading taken from the wells withcells cultured with the control medium was used as a 100% viability value. Thepercent viability was calculated by the formula
wastaken as the index of cell viability. The reading taken from the wells withcells cultured with the control medium was used as a 100% viability value. Thepercent viability was calculated by the formula