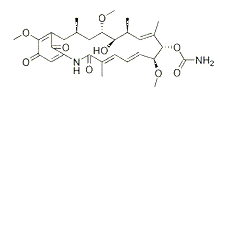
-
生物活性
Geldanamycin, a benzoquinone ansamycin, has been found to revert tyrosine kinase-induced oncogenic transformation. In the same study it was reported that Geldanamycin was able to bind elements of Hsp90 (heat shock protein 90) clients, such as HIF-1α (Hypoxia-inducible factor-1-α), and inhibit them. Binds to the ATP site of Hsp90 (Kd = 1.2 μM) and inhibits its chaperone activity. Geldanamycin binds in a specific and stable manner and as a result inhibits new protein maturation. Geldanamycin binds with high affinity into the ATP binding pocket of Hsp90. By binding them, Geldanamycin is thought to reduce new protein maturation and their downstream transcriptional activity. Geldanamycin has also displayed a capacity to bind to the endoplasmic reticulum homologue of the heat-shock protein, GP-96 and thus interferes with the cellular stress response. In addition, Geldanamycin has been reported as a potent inhibitor of the nuclear hormone receptor family. Other research has demonstrated Geldanamycin to be a protective agent against α-synuclein toxicity to dopaminergic neurons in Drosophila. Inhibits activities of oncogenic kinases (e.g. src, Raf), p53 and steroid receptors. Demonstrates antiproliferative effect on breast cancer stem-like cells.
-
体外研究
-
体内研究
-
激酶实验
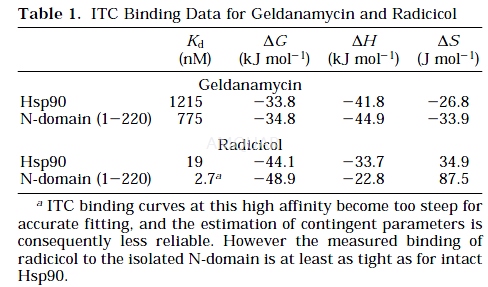
Isothermal Titration Calorimetry (ITC) ofNucelotide Binding[1]
The titration experiments were performedusing the MSC system. In each experiment, 16 aliquots of 15μLof radicicol (310μM in 1% DMSO) or geldanamycin (300μM in 1% DMSO)were injected into 1.3 mL of protein (31μM in 20 mM Tris-HCl,pH 7.5, 1mM EDTA) at 25°C, and the resulting data were fit after subtractingthe heats of dilution as described previously.15 Heats of dilution weredetermined in separate experiments from addition of radicicol or geldanamycininto buffer and buffer into protein. No evidence for binding of DMSO in thenucleotide binding site was observed. Titration data were fit using a nonlinearleast-squares curve-fitting algorithm with three floating variables: stoichiometry,binding constant (Kb =1/Kd), and change of enthalpy of interaction (ΔH°). Dissociation constants estimated for radicicol bind and forgeldanamycin binding to intact yeast Hsp90 are 19 nM and 1.22μM,respectively, and for binding to Hsp90 N-terminal domain are 2.7 nM and 0.78μM,respectively (see Table 1). No meaningful heats were observed with eithercompound binding to the C-terminal fragment.
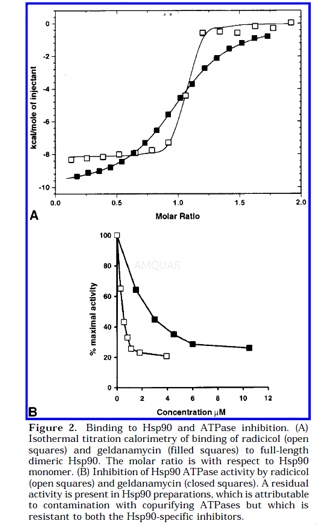
ATPaseAssay
The ATPase assay was based on aregenerating coupled enzyme assay in which the phosphorylation of ADP bypyruvate kinase at the expense of phosphoenolpyruvate is coupled to thereduction of the resulting pyruvate by lactate dehydrogenase at the expense ofNADH. Oxidation of NADH to NAD+ produces a loss of optical density at the NADH absorbancemaximum of 340nm, in direct stoichiometry to the amount of ADP phosphorylated.Each 1-mL assay contained 100mM Tris-HCl (pH 7.4), 20mM KCl, 6mM MgCl2,0.8mM ATP, 0.1mM NADH, 2 mM phosphoenolpyruvate, 0.2mg of pyruvate kinase, 0.05mg of Llactate dehydrogenase, and between 2 and 3.5nmol of Hsp90. SufficientNADH was added to give an initial absorbance of 0.3 at 340 nm prior to additionof Hsp90s or fragments, and activity was detected as a decrease in absorbance.Inhibition of ATPase activity by radicicol or geldanamycin was achieved by the additionof 1-10μL of antibiotic dissolved in 100% DMSO to a final concentrationof1.5, 9, 15, and 30μM. In control experiments, 1% DMSO present alone did not affect themeasured ATPase activities, and stoichiometric rephosphorylation of ADPdirectly added to the assay system was unaffected by 1% DMSO or by either ofthe antibiotics at all concentrations tested.
-
细胞实验
Cell line and culture conditions[2]
The human OS 143B cell line was cultured at37oC in a humidified atmosphere saturated with 5% CO2 using Dulbecco’s Modified Eagle’s medium supplemented with 10% heat-inactivatedfetal bovine serum and a penicillin (100 mg/mL)/ streptomycin (100 mg/mL)cocktail.
Celltreatment
Before each experiment, the cells weretrypsinized with a solution of 0.25% trypsin and 0.02% EDTA, and were cultured for24 h under the above-described conditions. The medium was then replaced withthe one containing geldanamycin (GA) according to an experimental design. Thestock solution of GA was prepared by dissolving the pure compound in DMSO. Thefinal concentration of DMSO in the working solution was ~0.001%. After an appropriateincubation time, the cells were harvested and analyzed by one of the methodsdescribed below.
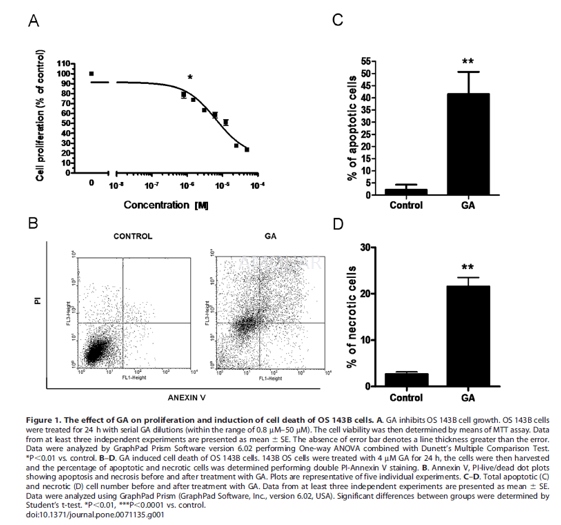
Cellviability assay (MTT assay)
OS 143B cells were seeded onto 96-wellplates at a density of ten thousand cells/well and cultured for 24 h. Mediumwas then removed and cells were treated with serial GA dilutions (within the rangeof 0.8μM–50μM) of GA for 24 h. After the appropriate incubation time, 0.5 mg/mLof 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) wasadded. The plates were then incubated at 37oC for 4 h and the supernatantwas removed. Finally, 100 mL of DMSO was added. Absorbance at 570 nm was determinedusing a microplate reader. The number of cells was calculated based onabsorbance values. The results were presented as a percentage of control. Each experimentwas performed at least three times.
Assessmentof apoptosis by flow cytometry with double Annexin V - propidium iodide (PI)staining
OS 143B cells were seeded onto six-wellplates at a density of three hundred thousand cells/well. After 24 h ofculturing in the standard medium, the cells were treated with GA for 24 h. The cellswere then pelleted and incubated with Annexin V and PI according tomanufacturer’s protocol.
Afterward, the cells (thirtythousand/sample) were analyzed, and the fluorescent signals of Annexin Vconjugate and PI were detected at the fluorescence intensity channels FL1 andFL3. The results were then analyzed by Cyflogic software, version 1.2.1. Eachexperiment was performed at least three times.
-
动物实验
Inoculation of kunming mice[3]
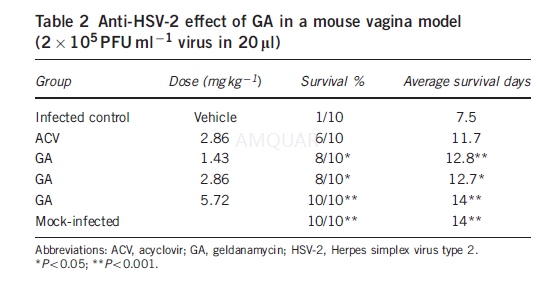
A mouse vaginal model of HSV-2 infectionwith modification was used to study in vivo anti-HSV-2 efficacy of GA.21Briefly, Mice vaginas were extensively cleaned by sterile cotton swab. A 20-mlsolution containing HSV-2 (2x105 PFU/ml) prepared in MEM wasinoculated intravaginally. The virus doses used in all infected experimentsresulted in a high rate of mortality (480%).
Antiviraleffect in vivo
In vagina model (10 mice per group), themice were treated intravaginally with three different concentrations of GAsuspension (5.72, 2.86 and 1.43mg /kg) 1 h post infection, three times a dayfor 7 days. The same procedure was applied to ACV group (2.86mg/kg) and theinfected control group (vehicle). Animals were monitored for morbidity andmortality for 14 days.
Virusshedding experiment
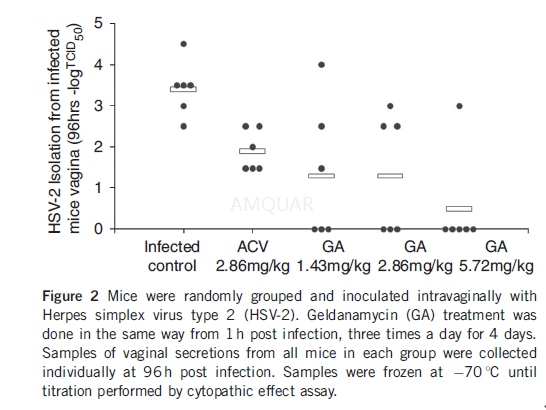
Mice were randomly grouped (six in eachgroup), color labeled and inoculated intravaginally with HSV-2. GA treatmentwas done in the same way as described above, except the duration of treatmentwas 4 days. Samples of vaginal secretions from all mice in each group werecollected individually at 96 h post infection. For sampling, the cotton sterileswab wetted by MEM were put into the vaginas for 5 s and very gently swabbed.Then the cotton swabs were put into a test tube with 1-ml MEM and antibiotics(penicillin 100U/ml, streptomycin 100 mg/ml) as soon as possible. Samples were frozenat -70 oC until titration was performed by cytopathic effect assay.
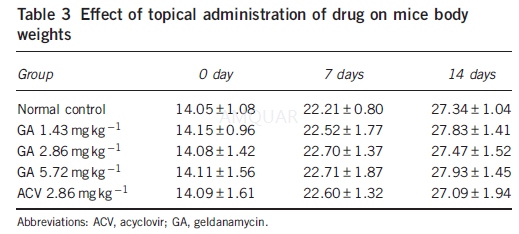
Drugtoxicity in vivo
Uninfected mice were grouped and treatedwith the same doses as the infected mice groups. Mice were weighed individuallyon days 0, 7 and 14.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Roe SM, P. C., O'Brien R, Ladbury JE, Piper PW, Pearl LH, Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 1999, 42 (2), 260-266.
[2] Gorska, M.; Marino Gammazza, A.; Zmijewski, M. A.; Campanella, C.; Cappello, F.; Wasiewicz, T.; Kuban-Jankowska, A.; Daca, A.; Sielicka, A.; Popowska, U.; Knap, N.; Antoniewicz, J.; Wakabayashi, T.; Wozniak, M., Geldanamycin-induced osteosarcoma cell death is associated with hyperacetylation and loss of mitochondrial pool of heat shock protein 60 (hsp60). PLoS One 2013, 8 (8), e71135.
[3] Li, Y. H.; Lu, Q. N.; Wang, H. Q.; Tao, P. Z.; Jiang, J. D., Geldanamycin, a ligand of heat shock protein 90, inhibits herpes simplex virus type 2 replication both in vitro and in vivo. J Antibiot (Tokyo) 2012, 65 (10), 509-12.
分子式
C29H40N2O9 |
分子量
560.64 |
CAS号
30562-34-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
10 mg/mL |
Water
Insoluble |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00019708 | Extranodal Marginal Zone B-cell Lymphoma of Mucosa-associated Lymphoid Tissue|Nodal Marginal Zone B-cell Lymphoma|Non-Hodgkin Lymphoma|Recurrent Adult Burkitt Lymphoma|Recurrent Adult Diffuse Large Cell Lymphoma|Recurrent Adult Diffuse Mixed Cell Lymphoma|Recurrent Adult Diffuse Small Cleaved Cell Lymphoma|Recurrent Adult Immunoblastic Large Cell Lymphoma|Recurrent Adult Lymphoblastic Lymphoma|Recurrent Grade 1 Follicular Lymphoma|Recurrent Grade 2 Follicular Lymphoma|Recurrent Grade 3 Follicular Lymphoma|Recurrent Marginal Zone Lymphoma|Recurrent Small Lymphocytic Lymphoma|Splenic Marginal Zone Lymphoma|Stage IV Adult Burkitt Lymphoma|Stage IV Adult Diffuse Large Cell Lymphoma|Stage IV Adult Diffuse Mixed Cell Lymphoma|Stage IV Adult Diffuse Small Cleaved Cell Lymphoma|Stage IV Adult Immunoblastic Large Cell Lymphoma|Stage IV Adult Lymphoblastic Lymphoma|Stage IV Grade 1 Follicular Lymphoma|Stage IV Grade 2 Follicular Lymphoma|Stage IV Grade 3 Follicular Lymphoma|Stage IV Mantle Cell Lymphoma|Stage IV Marginal Zone Lymphoma|Stage IV Small Lymphocytic Lymphoma|Unspecified Adult Solid Tumor, Protocol Specific | Drug: tanespimycin | National Cancer Institute (NCI) | Phase 1 | 1999-06-01 | 2013-12-13 |
| NCT00003969 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: tanespimycin | Cancer Research UK|National Cancer Institute (NCI) | Phase 1 | 1998-08-01 | 2013-06-25 |
| NCT00093405 | Kidney Cancer | Drug: tanespimycin | Memorial Sloan Kettering Cancer Center|National Cancer Institute (NCI) | Phase 2 | 2004-08-01 | 2013-06-21 |
| NCT00004065 | Bladder Cancer|Breast Cancer|Colorectal Cancer|Gastric Cancer|Head and Neck Cancer|Kidney Cancer|Leukemia|Lung Cancer|Melanoma (Skin)|Ovarian Cancer|Prostate Cancer|Unspecified Adult Solid Tumor, Protocol Specific | Drug: tanespimycin | Memorial Sloan Kettering Cancer Center|National Cancer Institute (NCI) | Phase 1 | 1999-07-01 | 2013-06-20 |
| NCT00087386 | Recurrent Melanoma|Stage III Melanoma|Stage IV Melanoma | Drug: tanespimycin|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2004-06-01 | 2013-04-09 |
| NCT00058253 | Recurrent Prostate Cancer|Stage IV Prostate Cancer|Unspecified Adult Solid Tumor, Protocol Specific | Drug: tanespimycin|Drug: docetaxel | National Cancer Institute (NCI) | Phase 1 | 2003-02-01 | 2014-06-16 |
| NCT00119236 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: tanespimycin|Drug: irinotecan hydrochloride | National Cancer Institute (NCI) | Phase 1 | 2005-05-01 | 2013-06-03 |
| NCT00319930 | Chronic Lymphocytic Leukemia | Drug: CNF1010 (17-AAG) | Biogen | Phase 1 | 2005-05-01 | 2010-03-04 |
| NCT00093821 | Childhood Chronic Myelogenous Leukemia|Childhood Desmoplastic Small Round Cell Tumor|Disseminated Neuroblastoma|Metastatic Childhood Soft Tissue Sarcoma|Metastatic Ewing Sarcoma/Peripheral Primitive Neuroectodermal Tumor|Metastatic Osteosarcoma|Previously Treated Childhood Rhabdomyosarcoma|Recurrent Childhood Acute Lymphoblastic Leukemia|Recurrent Childhood Acute Myeloid Leukemia|Recurrent Childhood Rhabdomyosarcoma|Recurrent Childhood Soft Tissue Sarcoma|Recurrent Ewing Sarcoma/Peripheral Primitive Neuroectodermal Tumor|Recurrent Neuroblastoma|Recurrent Osteosarcoma | Drug: tanespimycin|Other: pharmacological study|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1 | 2004-09-01 | 2013-06-03 |
| NCT01193491 | Hematologic Malignancies | Drug: IPI-493 | Infinity Pharmaceuticals, Inc. | Phase 1 | 2010-06-01 | 2015-04-14 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们