-
生物活性
Semaxanib is a cell-permeable ATP-competitive inhibitor of Flk-1 (VEGFR2/KDR, kinase insert domain receptor), which is the major growth factor for vascular endothelial cells. This receptor is a type III tyrosine kinase receptor that functions as the main mediator of VEGF-induced endothelial proliferation, survival, and migration. Studies suggest that Semaxanib also inhibits a variety of other receptors such as Met, Flt-3/Flk-2 (FLT-3 receptor tyrosine kinases), Ret, c-Kit, PDGFR, c-Abl and Arg (ABL), and ALK. In addition, Semaxanib regulates the signaling pathways used by oncogenic RET-transfected cells by regulating the extracellular signal regulated kinase (ERK) and JNK pathways. Inhibits tumor vascularization and growth of multiple tumor types.
-
体外研究
-
体内研究
-
激酶实验
Cellular Tyrosine Kinase Assays[1]
NIH 3T3 Flk-1 cells overexpressing Flk-1receptors were cultured in 10% heat-inactivated FCS in DMEM. NIH 3T3 Flk-1cells were seeded onto 96-well plates (2.5 x 104cells/well) in heatinactivated FCS/DMEM for 24 h. Serial dilutions of SU5416 were added, and thecells were further incubated for 2 h. Tyrosine phosphorylation was stimulatedby the addition of 500 ng/ml human recombinant VEGF for 5–10 min at 37°C, andthe phosphotyrosine content of the immunolocalized receptors was measured asdescribed previously using colorimetric or fluorescence anti-phosphotyrosineantibodies. Cellular kinase assays for PDGF and EGFR tyrosine kinase assayswere performed. For the measurement of insulin receptor kinase activity, NIH3T3 cells overexpressing the human insulin receptor (H25) were stimulated withinsulin. culture medium and cultured overnight, followed by serum depletion in0.1%
BiochemicalKinase Assays
Solubilized membranes from 3T3 Flk-1 cells wereadded to polystyrene ELISA plates that had been precoated with a monoclonalantibody that recognizes Flk-1. After an overnight incubation with lysate at4°C, serial dilutions of SU5416 were added to the immunolocalized receptor. Toinduce autophosphorylation of the receptor, various concentrations of ATP wereadded to the ELISA plate wells containing serially diluted solutions of SU5416.The autophosphorylation was allowed to proceed for 60 min at room temperatureand then stopped with EDTA. The amount of phosphotyrosine present on the Flk-1receptors in the individual wells was determined by incubating theimmunolocalized receptor with a biotinylated monoclonal antibody directedagainst phosphotyrosine. After removal of the unbound anti-phosphotyrosineantibody, avidin-conjugated horseradish peroxidase H was added to the wells. Astabilized form of 3,39,5,59-tetramethyl benzidine dihydrochloride and H2O2 was added to the wells. The color readout of the assay was allowed to developfor 30 min, and the reaction was stopped with H2SO4.Parallel biochemical kinase assays were performed to measure autophosphorylationon EGFR and fibroblast growth factor receptor.
-
细胞实验
Neuroblastoma Cell Culture and Treatments[2]
Human malignant neuroblastoma SH-SY5Y andSKN-BE2 cell lines were prepared. The SHSY5Y cells were cultured in 19 RPMI1640 medium while the SK-N-BE2 cells were cultured in 19 DMEM medium, bothsupplemented with 10% fetal bovine serum (FBS) and1% penicillin andstreptomycin. Cells were grown in 75-cm2 flasks and maintained in afully-humidified incubator containing 5% CO2 at 37 oC.
MTTColorometric Assay to Determine Residual Cell Viability
The 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) assay was used to performe the dose–response studies anddetermine the residual viability of cells after treatment with SU or EGCG aloneand in combination. Both SH-SY5Y and SK-N-BE2 cell lines were seeded at 3 x 105 cells/well in two 96-well plates separately and incubated overnight in afully-humidified incubator containing 5% CO2 at 37oC.Various concentrations of SU (5, 10, and 20μM) and EGCG(50, 100, and 200μM) and their respective combinations (SU + EGCG) were added to eachplate at 1-h interval in triplicates and incubated overnight. The MTT dyesolution (5 mg/ml) was prepared and added (10μl) to eachwell and the cells were incubated at 37oC for 3 h. Yellow color MTTwas reduced to purple color formazan crystals in living cells by themitochondrial enzyme system. Then, formazan crystals were dissolved by additionof 100μl of isopropyl alcohol and absorbance was measured at 570 nm wavelength using a plate reader. The data from the MTT assay were further analyzedto determine the efficacy of combination doses of two drugs using the Compusyn softwareto generate a combination index (CI). Conventionally, CI > 1 indicates antagonism,CI = 1 indicates additive effect, and CI < 1 indicates synergism of thecombination of drugs.

-
动物实验
Tumor-Bearing Mice Model[3]
Male BALB/c or C57BL6 mice (6–7 weeks) forthe inoculation of C26 or LLC and B16, respectively, were maintained at 25 oCand 55% of humidity with free access to standard chow and water. To preparetumor-bearing mice, one million tumor cells were subcutaneously injected in theback of mice.
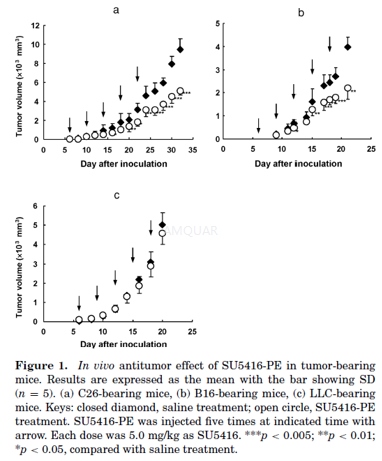
InVivo Antitumor Activity
When the tumor volume reached about 100 mm3 after the inoculation of C26, LLC, or B16 cells, saline or SU5416-PE (5 mg/kgas SU5416) was injected to mice of control or treatment group, respectively. Inaddition to this first injection, mice of control or treatment group were givenanother four injections of saline or SU5416-PE (5 mg/kg as SU5416 for eachdosing) at the predetermined times, respectively. The tumor volume was measuredevery other day with a caliper in two dimensions, and was calculated using thefollowing equation:
Tumor volume (mm3) = longerdiameter× (shorter one)2 × 0.52
A slope of tumor volume–time curve,representing the growth rate of each tumor in the treatment group (T), wasobtained during days 15–32, 10–20, or 12–21 for C26, B16, or LLC tumor,respectively, and divided by that in the control group (C) for each type oftumors to give an index (T/C) for the in vivo therapeutic effect for each tumor-bearingmouse model.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Fong TA, S. L., Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G, SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 1999, 59 (1), 99-106.
[2] Mohan, N.; Karmakar, S.; Banik, N. L.; Ray, S. K., SU5416 and EGCG work synergistically and inhibit angiogenic and survival factors and induce cell cycle arrest to promote apoptosis in human malignant neuroblastoma SH-SY5Y and SK-N-BE2 cells. Neurochem Res 2011, 36 (8), 1383-96.
[3] Ogawara, K.; Abe, S.; Un, K.; Yoshizawa, Y.; Kimura, T.; Higaki, K., Determinants for in vivo antitumor effect of angiogenesis inhibitor SU5416 formulated in PEGylated emulsion. J Pharm Sci 2014, 103 (8), 2464-9.
分子式
C15H14N2O |
分子量
238.28 |
CAS号
204005-46-9 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
10 mg/mL |
Water
Insoluble |
Ethanol
3 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00006247 | Brain and Central Nervous System Tumors | Drug: semaxanib | Pediatric Brain Tumor Consortium|National Cancer Institute (NCI) | Phase 1 | 2000-08-01 | 2009-10-13 |
| NCT00009919 | Recurrent Renal Cell Cancer|Stage IV Renal Cell Cancer | Drug: semaxanib | National Cancer Institute (NCI) | Phase 2 | 2000-12-01 | 2013-01-22 |
| NCT00005642 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: semaxanib | Case Comprehensive Cancer Center|National Cancer Institute (NCI) | Phase 1 | 2000-05-01 | 2010-06-09 |
| NCT00006003 | Recurrent Melanoma|Stage IV Melanoma | Drug: semaxanib|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2000-07-01 | 2013-01-23 |
| NCT00005647 | Head and Neck Cancer | Drug: paclitaxel|Drug: semaxanib | Case Comprehensive Cancer Center|National Cancer Institute (NCI) | Phase 1 | 2000-05-01 | 2010-06-09 |
| NCT00005942 | Chronic Myelomonocytic Leukemia|Previously Treated Myelodysplastic Syndromes|Recurrent Adult Acute Myeloid Leukemia|Refractory Anemia With Excess Blasts|Refractory Anemia With Excess Blasts in Transformation | Drug: liposomal daunorubicin citrate|Drug: semaxanib|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1|Phase 2 | 2000-03-01 | 2013-01-22 |
| NCT00005818 | Adenocarcinoma of the Colon|Adenocarcinoma of the Rectum|Recurrent Colon Cancer|Recurrent Rectal Cancer|Stage III Colon Cancer|Stage III Rectal Cancer|Stage IV Colon Cancer|Stage IV Rectal Cancer | Drug: irinotecan hydrochloride|Drug: semaxanib|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1|Phase 2 | 2000-03-01 | 2013-01-22 |
| NCT00006014 | Malignant Mesothelioma | Drug: semaxanib | National Cancer Institute (NCI) | Phase 2 | 2000-08-01 | 2013-05-31 |
| NCT00006001 | Colorectal Cancer | Drug: semaxanib | National Cancer Institute (NCI) | Phase 2 | 2000-08-01 | 2013-05-31 |
| NCT00005862 | Gastrointestinal Stromal Tumor|Sarcoma | Drug: semaxanib | National Cancer Institute (NCI) | Phase 2 | 2000-10-01 | 2013-02-08 |
| NCT00005822 | Breast Cancer | Drug: doxorubicin hydrochloride|Drug: semaxanib|Procedure: conventional surgery|Radiation: radiation therapy|Drug: tamoxifen | Case Comprehensive Cancer Center|National Cancer Institute (NCI) | Phase 1 | 2000-04-01 | 2010-06-10 |
| NCT00006013 | Multiple Myeloma and Plasma Cell Neoplasm | Drug: semaxanib | Fox Chase Cancer Center|National Cancer Institute (NCI) | Phase 2 | 2000-06-01 | 2013-07-10 |
| NCT00005042 | Sarcoma | Drug: semaxanib | AIDS Malignancy Consortium|National Cancer Institute (NCI) | Phase 2 | 2000-01-01 | 2014-08-28 |
| NCT00006361 | Carcinoma of Unknown Primary|Head and Neck Cancer|Non-melanomatous Skin Cancer | Drug: semaxanib | Memorial Sloan Kettering Cancer Center|National Cancer Institute (NCI) | Phase 2 | 2000-12-01 | 2009-12-03 |
| NCT00026260 | Cervical Cancer | Drug: semaxanib | Gynecologic Oncology Group|National Cancer Institute (NCI) | Phase 2 | null | 2013-06-20 |
| NCT00004868 | Brain and Central Nervous System Tumors | Drug: semaxanib | North American Brain Tumor Consortium|National Cancer Institute (NCI) | Phase 1|Phase 2 | 2000-02-01 | 2008-11-22 |
| NCT00026377 | Prostate Cancer | Drug: bicalutamide|Drug: flutamide|Drug: goserelin acetate|Drug: leuprolide acetate|Drug: SU5416|Radiation: radiation therapy | University of Chicago|National Cancer Institute (NCI) | Phase 1 | 2001-11-01 | 2013-09-04 |
| NCT00017316 | Melanoma (Skin) | Drug: semaxanib|Drug: thalidomide | National Cancer Institute (NCI) | Phase 2 | 2001-03-01 | 2013-02-08 |
| NCT00006002 | Prostate Cancer | Drug: dexamethasone|Drug: semaxanib | University of Chicago|National Cancer Institute (NCI) | Phase 2 | 2000-06-01 | 2013-09-04 |
| NCT00005931 | Sarcoma, Kaposi|HIV Infections | Drug: SU5416 | SUGEN|NIH AIDS Clinical Trials Information Service | Phase 2 | null | 2005-06-23 |
| NCT00006257 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: paclitaxel|Drug: semaxanib | City of Hope Medical Center|National Cancer Institute (NCI) | Phase 1 | 2000-11-01 | 2010-01-11 |
| NCT00006384 | Kidney Cancer | Biological: recombinant interferon alfa|Drug: semaxanib | City of Hope Medical Center|National Cancer Institute (NCI) | Phase 2 | 2000-11-01 | 2011-10-18 |
| NCT00006155 | Fallopian Tube Neoplasm|Ovarian Cancer|Peritoneal Neoplasm | Drug: SU5416 and carboplatin | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 | 2000-08-01 | 2008-03-03 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们