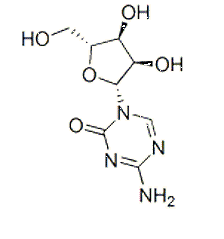
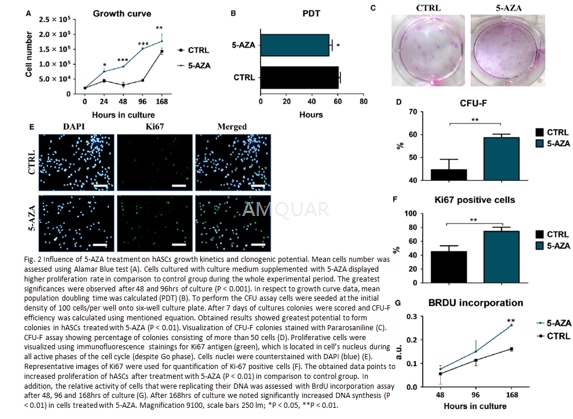
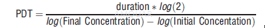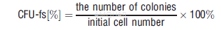| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00901537 | Non-small Cell Lung Cancer|Squamous Cell Carcinoma of the Head and Neck | Drug: Azacitidine and Cisplatin | Loma Linda University | Phase 1 | 2009-02-01 | 2014-06-02 |
| NCT01488565 | Myelodysplastic Syndromes (MDS)|Acute Myeloid Leukaemia (AML) | Drug: Azacitidine and eltrombopag | Peter MacCallum Cancer Centre, Australia|GlaxoSmithKline|Celgene Corporation | Phase 2 | 2010-12-01 | 2016-02-12 |
| NCT02940483 | Brain Tumor Recurrent | Drug: 5-Azacytidine | The University of Texas Health Science Center, Houston | Early Phase 1 | 2016-12-01 | 2016-12-09 |
| NCT01305460 | Myelodysplastic Syndrome | Drug: Azacitidine | Groupe Francophone des Myelodysplasies | Phase 1|Phase 2 | 2011-07-01 | 2015-10-28 |
| NCT02038816 | Myelodysplastic Syndromes | Drug: Deferasirox + Azacitidine|Drug: Azacitidine | Sunnybrook Health Sciences Centre|Novartis | Phase 2 | 2014-03-01 | 2014-01-15 |
| NCT02985190 | MDS|Systemic Autoimmune Diseases | Drug: Azacitidine | Groupe Francophone des Myelodysplasies|Celgene | Phase 2 | 2016-12-01 | 2016-12-02 |
| NCT01305135 | High Grade Myelodysplastic Syndrome Lesions | Drug: azacitidine and idarubicin | Groupe Francophone des Myelodysplasies | Phase 1|Phase 2 | 2010-12-01 | 2015-10-28 |
| NCT01379274 | MDS | Drug: Lenalidomide and azacitidine combination | Rush University Medical Center|Celgene Corporation | Phase 2 | 2011-01-01 | 2013-11-26 |
| NCT01537744 | Solid Tumors|Virally Mediated Cancers and Liposarcoma | Drug: oral 5-azacitidine in combination with romidepsin | Sidney Kimmel Comprehensive Cancer Center|Celgene Corporation | Phase 1 | 2012-02-01 | 2017-03-21 |
| NCT02323139 | MDS | Drug: Azacitidine and LDE255 | Groupe Francophone des Myelodysplasies|Novartis | Phase 1 | 2015-02-01 | 2016-05-30 |
| NCT01599325 | Myelodysplastic Syndrome (MDS) | Drug: Azacitidine | Celgene | Phase 2 | 2012-07-01 | 2017-01-30 |
| NCT01462578 | Acute Myelocytic Leukemia|Myelodysplastic Syndrome | Drug: Azacitidine | Technische Universit盲t Dresden | Phase 2 | 2011-09-01 | 2016-02-09 |
| NCT01324960 | Myelodysplastic Syndromes | Drug: Ceplene庐, IL-2, Azacitidine|Drug: Azacitidine | Groupe Francophone des Myelodysplasies|EpiCept Corporation | Phase 1|Phase 2 | 2011-03-01 | 2014-03-19 |
| NCT00795548 | Myelodysplastic Syndrome|Acute Myeloid Leukemia | Drug: 5-Azacitidine | Heinrich-Heine University, Duesseldorf | Phase 2 | 2008-11-01 | 2012-01-20 |
| NCT00413478 | Chronic Lymphocytic Leukemia|Leukemia | Drug: 5-Azacytidine | M.D. Anderson Cancer Center|Celgene Corporation | Phase 2 | 2006-09-01 | 2015-06-26 |
| NCT00387647 | Leukemia | Drug: Azacitidine | H. Lee Moffitt Cancer Center and Research Institute|Celgene Corporation | Phase 2 | 2006-08-01 | 2014-08-19 |
| NCT02196857 | Leukemia | Drug: Azacytidine|Drug: Sorafenib | M.D. Anderson Cancer Center|Bayer | Phase 2 | 2015-02-01 | 2017-03-09 |
| NCT01038635 | Leukemia | Drug: 5-Azacytidine|Drug: Lenalidomide | M.D. Anderson Cancer Center|Celgene | Phase 1|Phase 2 | 2009-12-01 | 2016-12-19 |
| NCT00350818 | Myelodysplastic Syndrome|Leukemia | Drug: Azacitidine | M.D. Anderson Cancer Center|Celgene Corporation | Phase 1 | 2005-10-01 | 2012-07-27 |
| NCT00721214 | Myelodysplastic Syndrome | Drug: 5-azacytidine | Virginia Commonwealth University|Celgene Corporation | Phase 2 | 2008-07-01 | 2016-02-03 |
| NCT01913951 | Myelodysplastic Syndromes | Drug: vosaroxin|Drug: Azacitidine | Washington University School of Medicine | Phase 1 | 2013-11-22 | 2017-02-18 |
| NCT00728520 | Acute Myeloid Leukemia|Elderly | Drug: Azacitidine | Kansas City Veteran Affairs Medical Center | Phase 2 | 2008-07-01 | 2008-08-05 |
| NCT01652781 | Myelodysplastic Syndrome | Drug: Azacitidine | Seoul St. Mary's Hospital|Celgene Corporation | Phase 2 | 2012-03-01 | 2015-11-18 |
| NCT02399917 | Leukemia | Drug: 5-azacytidine|Drug: Lirilumab|Behavioral: Phone Call | M.D. Anderson Cancer Center|Bristol-Myers Squibb | Phase 2 | 2015-04-01 | 2017-03-07 |