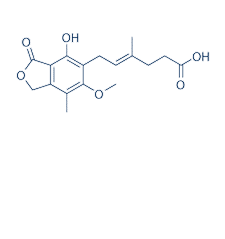
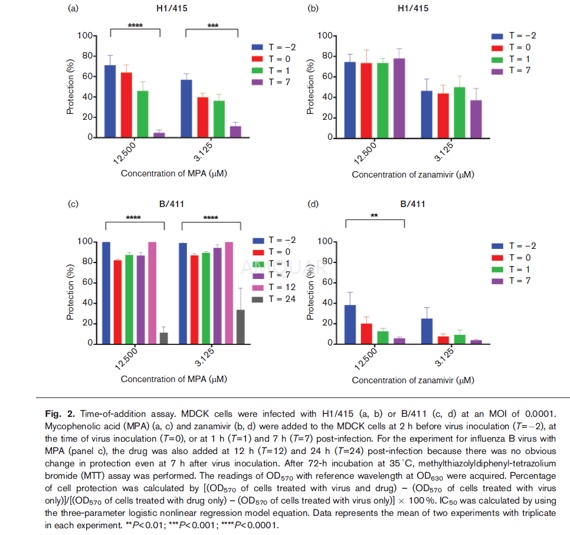
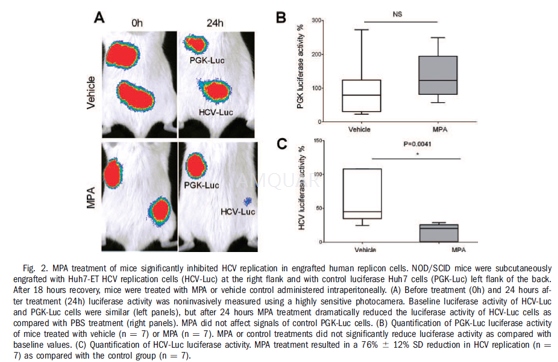
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01336296 | Kidney Transplant Recipients | Drug: mycophenolic acid | University of Cincinnati|Novartis Pharmaceuticals | Phase 4 | 2010-09-01 | 2016-07-05 |
| NCT00374803 | End Stage Renal Disease (ESRD) | Drug: Mycophenolic Acid (Myfortic) | University of Cincinnati|Novartis | Phase 4 | 2006-04-01 | 2016-03-28 |
| NCT00351377 | Autoimmune Disease | Drug: Enteric-coated Mycophenolate Sodium | Novartis Pharmaceuticals|Novartis | Phase 3 | 2006-06-01 | 2011-04-19 |
| NCT01053221 | Kidney Transplantation | Drug: Mycophenolic Acid|Drug: Standard of Care: CNI and MPA | University of Wisconsin, Madison | | 2006-03-01 | 2017-02-16 |
| NCT00542763 | Primary Sjogren's Syndrome | Drug: Mycophenolate sodium | University Hospital Muenster|Novartis | Phase 1 | 2005-04-01 | 2007-10-10 |
| NCT01042457 | Lupus Nephritis | Drug: Mycophenolate mofetil | Chulalongkorn University | Phase 3 | 2009-05-01 | 2013-09-27 |
| NCT00585468 | Kidney Transplantation | Drug: Myfortic | University of Utah | Phase 4 | 2007-12-01 | 2016-04-01 |
| NCT02630563 | Pediatric Liver Transplantation | Drug: Corticosteroids|Drug: Cyclosporine|Drug: mycophenolate mofetil | Hoffmann-La Roche | Phase 2 | 2003-05-01 | 2016-04-19 |
| NCT00369278 | Renal Transplantation | Drug: Enteric-coated mycophenolate sodium (EC-MPS) | Novartis Pharmaceuticals|Novartis | Phase 3 | 2006-06-01 | 2011-03-25 |
| NCT00239005 | Renal Transplant | Drug: Enteric-Coated Mycophenolate Sodium (EC-MPS)|Drug: Mycophenolate Mofetil (MMF) | Novartis Pharmaceuticals|Novartis | Phase 4 | 2005-09-01 | 2011-02-23 |
| NCT02370693 | Interstitial Lung Disease|ILD|Systemic Sclerosis|Scleroderma | Drug: Bortezomib|Drug: Placebo|Drug: Mycophenolate mofetil | Northwestern University|National Heart, Lung, and Blood Institute (NHLBI) | Phase 2 | 2016-03-01 | 2017-01-20 |
| NCT01487577 | Allogeneic Blood and Marrow Transplantation (BMT)|Graft Versus Host Disease | Drug: Mycophenolate mofetil | University of Pittsburgh | Phase 2 | 2010-06-01 | 2016-05-13 |
| NCT00619216 | GI Disturbance | Drug: Mycophenolic Acid (Myfortic) | University of North Carolina, Chapel Hill|Novartis Pharmaceuticals | | 2008-03-01 | 2011-06-01 |
| NCT00997412 | Myasthenia Gravis | Drug: Mycophenolic acid|Drug: AZA | Qualitix Clinical Research Co., Ltd. | | 2009-05-01 | 2009-10-16 |
| NCT00991510 | Stable Renal Transplant Recipients | Drug: mycophenolate mofetil (Myfenax)|Drug: mycophenolate mofetil (Cellcept) | Teva Pharmaceutical Industries|Parexel | Phase 4 | 2009-08-01 | 2013-10-29 |
| NCT02435368 | Lupus Nephritis | Drug: mycophenolic acid | The Third Affiliated Hospital of Southern Medical University | | 2015-04-01 | 2015-04-30 |
| NCT01711489 | Healthy Subjects|Pharmacokinetics of Isavuconazole|Pharmacokinetics of Plasma Mycophenolic Acid (MPA)|Pharmacokinetics of Plasma Phenolic Glucuronide of MPA (MPAG) | Drug: isavuconazole|Drug: Mycophenolate mofetil | Astellas Pharma Global Development, Inc.|Basilea Pharmaceutica International Ltd|Astellas Pharma Inc | Phase 1 | 2012-03-01 | 2012-10-18 |
| NCT01467011 | End Stage Liver Disease | Drug: Enteric-coated Mycophenolate Sodium | R. Mark Ghobrial, MD|Novartis|The Methodist Hospital System | Phase 4 | 2010-12-01 | 2016-11-01 |
| NCT02743247 | Healthy Volunteers | Drug: Tacrolimus|Drug: Mycophenolate mofetil | Seoul National University Hospital|Ministry of Food and Drug Safety, Korea | Phase 1 | 2015-10-01 | 2016-04-14 |
| NCT00451867 | Interstitial Cystitis|Painful Bladder Syndrome | Drug: Mycophenolate Mofetil|Drug: Mycofenolate Mofetil (MMF)|Drug: Placebo | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Phase 3 | 2007-03-01 | 2010-01-12 |