-
生物活性
SCH772984, a novel and selective inhibitorof ERK1/2 that displays behaviors of both type I and type II kinase inhibitors. SCH772984, identified by an affinity-based mass spectroscopy high-throughput platform, is a novel, potent and ATP-competitive inhibition of ERK1 and ERK2 with 50% inhibition concentration IC50 values of 4 nmol/L and 1 nmol/L respectively. Although it displays behaviors of both type I and type II kinase inhibitors, SCH772984 is highly selective against only seven kinases, including CLK2, FLT4, GSG2, MAP4K4, MAPK1, MINK1, PRKD1 and TTK, out of a wide range of 300 tested with more than 50% inhibition at a concentration of 1 μmol/L. Study results have shown that SCH772984 potently inhibits tumor cells with mutations in BRAF, NRAS and KRAS at nanomolar concentrations.
-
体外研究
-
体内研究
5% DMSO+Corn oil
-
激酶实验
ERK2 IMAP Enzymatic Assay[1]
SCH772984 was tested in 8-point dilutioncurves in duplicate against purifi ed ERK1 or ERK2. The enzyme was added to thereaction plate and incubated with the compound before adding a solution of substratepeptide and ATP. Fourteen microliters of diluted enzyme (0.3 ng active ERK2 perreaction) was added to each well of a 384-well plate. The plates were gentlyshaken to mix the reagents and incubated for 45 minutes at room temperature.The reaction was stopped with 60 μL of IMAP Binding Solution (1:2,200 dilutionsof IMAP beads in 1× binding buffer). The plates were incubated at room temperaturefor an additional 0.5 hours to allow complete binding of phosphopeptides to theIMAP beads. Plates were read on the LJL Analyst.

-
细胞实验
Reagents and cell lines[2]
SCH722984, MK-2206 (AKT inhibitor), MK-8669 (mTOR inhibitor), vemurafenib andtrametinib were purchased. All drugs were reconstituted in100% dimethylsulfoxide (DMSO) to a final concentration of 10 mM. Human melanoma cell lineswere cultured in RPMI 1640 with L-glutamine, 10% fetal bovine serum (FBS), and1% penicillin, streptomycin and fungizone. Cultures were incubated in a water saturatedincubator at 37°C with 5% CO2.
Cellviability
All cell lines were treated in duplicateswith 0–10 μM of SCH722984, vemurafenib and trametinib alone or in combinationand constant amount of DMSO for all the conditions. After incubation for 72–120hours, the cell viability was determined using CellTiter-Glo Luminescent CellViability Assay, an ATP based bioluminescent assay, as per manufacturer’s instructions.Each experiment was repeated independently at least 3 times.
For long-term culture experiments, M238 andM792 cell lines were plated at 20,000 cells per well in 96-well plates andchronically exposed to 500nM of vemurafenib, SCH722984 or the combination.Cells were re-fed with fresh media containing the appropriate inhibitor(s) weekly.Pictures were taken weekly to document visual differences in betweenconditions. Plates were considered for cell viability reading with[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) when several wellsappeared visually to be >90% confluent. The number of viable cells in eachwell was determined based on a standard curve of known cell numbers. Eachcondition was repeated in triplicate independent experiments.
Westernblotting
All melanoma cells were washed withice-cold phosphate buffered saline (PBS) twice and lysed with RIPA buffercontaining phosphatase and protease inhibitor. Protein extracts were separatedwith SDS-PAGE in 4-12% tris-glycine gels and transferred to immun-blot PVDFmembrane. After blocking for 1 hour in PBS containing 0.1% Tween 20 and 5%nonfat milk or 5% bovine serum albumin (BSA) in PBS, the membrane was exposedto various primary antibodies overnight, followed by secondary antibodies conjugatedto horseradish peroxidase. ECL-Plus kit was used to check immunoreactivity andblots were scanned using a Typhoon scanner. Primary antibodies included pERKThr202/Tyr204, total ERK, pMEK Ser217/221, total MEK, pAKT Ser473, total AKT,pRSK, total RSK, beta-actin.
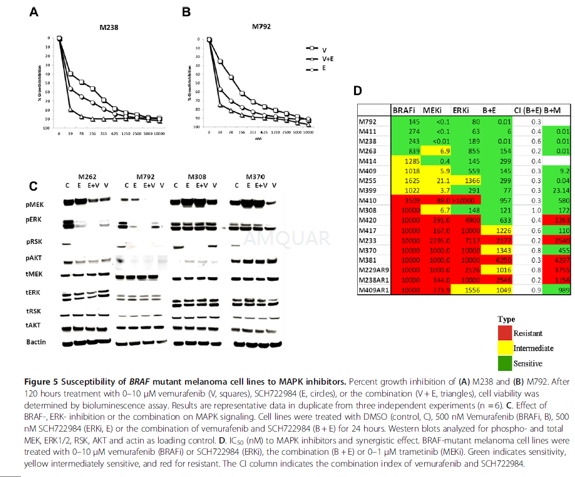
-
动物实验
Experimental animal[3]
Adult female Sprague–Dawley rats, 200–220g, were used. Experiments would be carried out after rats’ one-week adaption tothe environment. Light time in the laboratory is from8:00 to 20:00, temperature20 to 25oC, and humidity 40 to 60%. Rats are free to get food andwater.
Spinalcord subarachnoid catheterization
Rats are intraperitoneally injected with10% chloral hydrate (0.3 ml/100g), and after being anaesthetized, subarachnoid catheterwould be put. With animals prone, roll out a cloth under the abdomen to supportthe back, longitudinally cut for 1 cm at spinous process gap L6S1 afterdisinfection, then cut up the skin and fascia superficialis, reveal spinousprocess gap after muscle with blunt dissention, slowly push Catheter PE-10 ofbuilt-in guide silk, with sense of loose, and the rat tail can be seenjittering that the vertebral tube has been into it. Then take out the guidesilk, slowly put Catheter PE-10 into cavum subarachnoidale, with cerebrospinalfluid clearly spilling, determining the depth which is 3 cm, then suture andfix near end catheter. Lead lateral ends of the catheter to the back of theneck through a hole under skin with epidural puncture needle, 2 cm outside,suture and fix, and seal the catheter with hot melt. Feed in a single cageafter operation. Observe rats’ behaviors after their waking up for 24 h, andthose with paralysis, lameness, and dystropy would be given up; intrathecallyinject 20 ll lidocaine, and within 30 s no one has paraplegia.
Establishmentof bone cancer pain model
Establish rats’ tibia bone cancer pianmodel. Select rats recovering well and meeting experimental requirements withan intrathecal tube, anesthetize them with 10% chloral hydrate (0.3 ml/100 g),make them supine, fixed, right hind leg clipped, skin disinfection, cut about0.5 cm incision at the top of tibia, expose tibia, and make a hole with 1 mlsyringe needles. A sense of breakthrough proves the needle has thorn intomarrow cavity, then push microsyringe into marrow cavity while pulling out theneedle, then slowly pull out the needle, and red spinal cord can be seen, whichshows that the needle has thorn into marrow cavity. Slowly inject 10μlcell suspension (containing cell number 100000) with Walker256 for 1 min, pullout microsyringe after 2 min, and quickly seal the pinhole with medical glue,then bond skin.
Grouping
40 SD rats with intrathecal tube arerandomly divided into 5 groups (n =8): sham group and bone cancer pain group(Group BCP + DMSO, Group BCP + SCH0.1 Group BCP+SCH1.0 and Group BCP +SCH10).Sham group is injected with 10μgSCH772984 intrathecally, and bone cancer pain group is injected with 10μl5%DMSO, 0.1μg SCH, 1.0μg SCH, 10μg SCH (SCH dissolved in 10μl 5% DMSO) intrathecally.
Behavioraldetermination index
Before and after modeling 3, 6, 9d,determine the rats’ mechanical withdrawal threshold (MWT) 1 h before medicationand 1, 3, 6, 9, 12, 15, 18, 24 h after medication, as well as 2-min spontaneouspaw withdrawal.
Determinationof MWT
Mechanical hyperalgesia is determined byelectronic Von Frey tenderness instrument. Place a plexiglass box (22 cm x 12cm x 22 cm) on a metal sifter, put rats in for30 min, and stimulate centralarea of right rear foot with Von Frey. When rats lift foot, shrink foot or evenfast record numbers on electronic screen which is MWT (g). Continuously measure5 times with 10 s for each interval, and then take average value.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Morris EJ JS, Restaino CR, Dayananth P, Zhu H, Cooper A, Carr D, Deng Y, Jin W, Black S, Long B, Liu J, Dinunzio E, Windsor W, Zhang R, Zhao S, Angagaw MH, Pinheiro EM, Desai J, Xiao L, Shipps G, Hruza A, Wang J, Kelly J, Paliwal S, Gao X, Babu BS, Zhu L, Daublain P, Zhang L, Lutterbach BA, Pelletier MR, Philippar U, Siliphaivanh P, Witter D, Kirschmeier P, Bishop WR, Hicklin D, Gilliland DG, Jayaraman L, Zawel L, Fawell S, Samatar AA. Discovery of a novel ERK inhibitor with activity in mode
[2] Wong DJ RL, Atefi MS, Lassen A, Avarappatt G, Cerniglia M, Avramis E, Tsoi J, Foulad D, Graeber TG, Comin-Anduix B, Samatar A, Lo RS, Ribas A. Antitumor activity of the ERK inhibitor SCH772984 [corrected] against BRAF mutant, NRAS mutant and wild-type melanoma. Mol Cancer. 2014;13:194.
[3] Bian J ZS, Ma W, Li C, Ashraf MA. Analgesic effect and possible mechanism of SCH772984 intrathecal injection on rats with bone cancer pain. Saudi Pharm J. 2016;24(3):354-362.
分子式
C33H33N9O2 |
分子量
587.67 |
CAS号
942183-80-4 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
50 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们




















