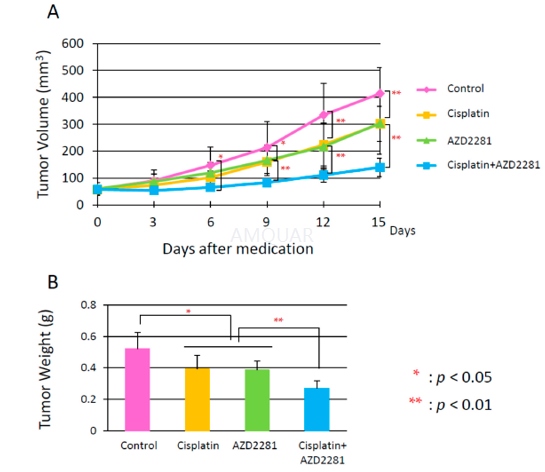
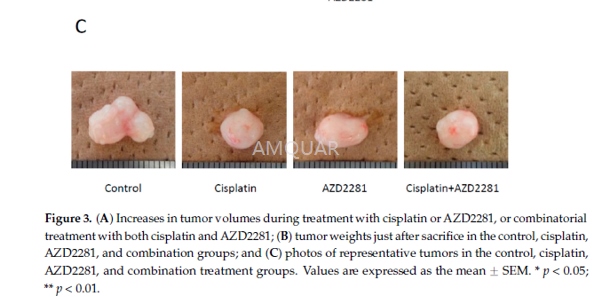
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01562210 | NSCLC | Drug: Olaparib|Drug: Cisplatin|Radiation: Radiation | The Netherlands Cancer Institute|AstraZeneca | Phase 1 | 2012-04-01 | 2016-12-07 |
| NCT02677038 | Pancreatic Cancer | Drug: Olaparib | M.D. Anderson Cancer Center|AstraZeneca | Phase 2 | 2016-11-01 | 2017-01-04 |
| NCT01682772 | Adenocarcinoma of the Prostate | Drug: Olaparib | Institute of Cancer Research, United Kingdom|Royal Marsden NHS Foundation Trust | Phase 2 | 2012-07-01 | 2014-10-02 |
| NCT02533765 | Neoplasms, Germ Cell and Embryonal | Drug: Olaparib | Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumori | Phase 2 | 2015-05-01 | 2016-09-28 |
| NCT02338622 | Advanced Cancer | Drug: olaparib|Drug: AZD5363 | Royal Marsden NHS Foundation Trust|Institute of Cancer Research, United Kingdom|AstraZeneca | Phase 1 | 2014-03-01 | 2015-01-16 |
| NCT03047135 | Prostate | Drug: Olaparib | Sidney Kimmel Comprehensive Cancer Center | Phase 2 | 2017-03-01 | 2017-02-10 |
| NCT01813474 | Cancer|Advanced Solid Malignancies | Drug: olaparib | AstraZeneca | Phase 1 | 2013-03-01 | 2016-10-20 |
| NCT02227082 | Locally Advanced Malignant Neoplasm|Inflammatory Breast Carcinoma|Triple-Negative Invasive Breast Carcinoma | Radiation: radiotherapy|Drug: olaparib | The Netherlands Cancer Institute | Phase 1 | 2013-10-01 | 2016-09-14 |
| NCT02229656 | Laryngeal Cancer Stage II|Laryngeal Cancer Stage III|Carcinoma, Squamous Cell|Head and Neck Neoplasms | Radiation: radiotherapy|Drug: Olaparib | The Netherlands Cancer Institute|AstraZeneca | Phase 1 | 2014-02-01 | 2016-09-14 |
| NCT01623349 | Ovarian Cancer|Breast Cancer | Drug: BKM120 and Olaparib|Drug: BYL719 and Olaparib | Dana-Farber Cancer Institute|Novartis|AstraZeneca | Phase 1 | 2012-09-01 | 2017-03-22 |
| NCT01583543 | Ewing's Sarcoma | Drug: Olaparib | Massachusetts General Hospital | Phase 2 | 2012-05-01 | 2016-03-08 |
| NCT02032823 | Breast Cancer | Drug: Olaparib|Drug: Placebo | AstraZeneca|Breast International Group|Frontier Science & Technology Research Foundation, Inc.|NRG Oncology|Myriad Genetic Laboratories, Inc.|Br.E.A.S.T. -Data Center & Operational Office Institut Jules Bordet | Phase 3 | 2014-04-01 | 2017-02-20 |
| NCT02476968 | BRCA or HRR+ Mutated Ovarian Cancer Patients | Drug: Olaparib | AstraZeneca | Phase 4 | 2015-09-28 | 2017-03-06 |
| NCT02681562 | Breast Cancer|Triple Negative Breast Cancer | Drug: olaparib | Istituti Ospitalieri di Cremona | Phase 2 | 2016-01-01 | 2016-02-12 |
| NCT02398058 | Soft Tissue Sarcoma|Bone Tumor | Drug: trabectedin|Drug: olaparib | Italian Sarcoma Group|PharmaMar|AstraZeneca|Istituto Di Ricerche Farmacologiche Mario Negri | Phase 1 | 2014-10-01 | 2017-03-07 |
| NCT01758731 | Squamous Cell Carcinoma of the Head and Neck | Drug: Olaparib|Drug: Cetuximab|Radiation: Radiation Therapy | University of Colorado, Denver | Phase 1 | 2012-07-09 | 2017-02-06 |
| NCT02855697 | Ovarian Cancer | Drug: Olaparib|Drug: Cediranib|Drug: Platinum-based Chemotherapy | Anna Thomason|The Christie NHS Foundation Trust | Early Phase 1 | 2016-11-01 | 2016-08-25 |
| NCT01929603 | Solid Tumours | Procedure: Pharmacokinetic sampling|Drug: Rifampicin|Drug: Olaparib tablet dosing | AstraZeneca | Phase 1 | 2013-12-01 | 2017-01-13 |
| NCT02446704 | Small Cell Lung Cancer | Drug: Olaparib|Drug: Temozolomide | Massachusetts General Hospital|AstraZeneca | Phase 1|Phase 2 | 2015-09-01 | 2017-02-24 |
| NCT02511795 | Ovarian, Breast, and Second-Line Small Cell Lung Cancer | Drug: AZD1775|Drug: Olaparib | AstraZeneca | Phase 1 | 2015-08-01 | 2017-02-23 |
| NCT02506816 | Endometrial Carcinoma | Drug: Olaparib | MedSIR|AstraZeneca|Experior | Early Phase 1 | 2016-02-01 | 2016-11-07 |