-
生物活性
Tariquidar is a potent and selective noncompetitive inhibitor of P-glycoprotein with Kd of 5.1 nM, reverses drug resistance in MDR cell Lines. Tariquidar displays high-affinity binding to P-gp with Bmax of 275 pmol/mg. Tariquidar shows non-competitive interaction with the P-gp substrates vinblastine and paclitaxel. Tariquidar is able to inhibit the vanadate-sensitive ATPase activity of P-gp by 60-70%, with potent IC50 values of 43 nM. Tariquidar may inhibit other resistance mechanisms at higher concentrations. 1 μM Tariquidar abrogates ABCG2 (BCRP)-mediated resistance to camptothecins in vitro. Tariquidar potentiates the cyto-toxicity of several drugs including doxorubicin, paclitaxel, etoposide, and vincristine; complete reversal of resistance is achieved in the presence of 25- 80 nM Tariquidar.
-
体外研究
-
体内研究
30% propylene glycol, 5% Tween 80, 65% D5W(5%葡萄糖水溶液)
-
激酶实验
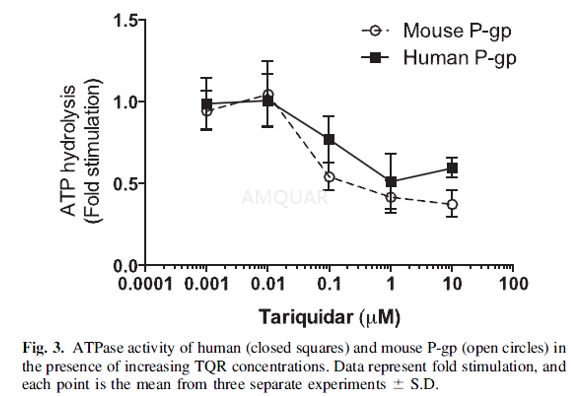
ATPase Assays[1]
To determine whether tariquidar (TQR)affects human and mouse P-gp differently, ATPase assays were performed usingcrude membrane extracts from High-five insect cells expressing either human ormouse P-gp. Due to the fact that wild-type mouse mdr1a cDNA is toxic in thebacterial cells needed for the cloning process, an M107L point mutation wasintroduced, which reduced bacterial toxicity but retained functionality. Themembrane vesicles in ATPase assay buffer [50mM MES-Tris buffer (pH 6.8), 50mM KCl,5mM sodiumazide, 1mM EGTA, 1mM ouabain, 10mM MgCl2, and 2mMdithiothreitol were incubated in varying concentrations of TQR with or without0.3mM sodium orthovanadate. ATP hydrolysis was measured by estimating therelease of inorganic phosphate after incubation with 5mM ATP.
-
细胞实验
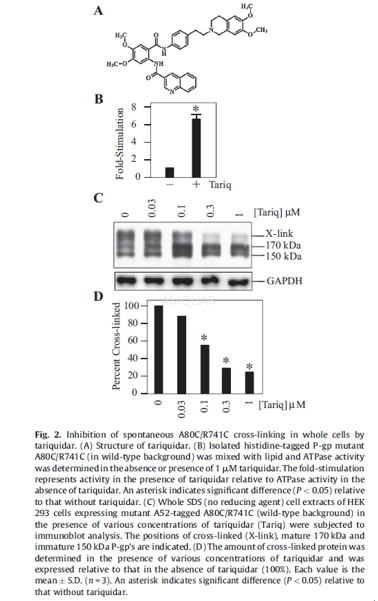
Effect of substrates and inhibitors onA80C/R741C cross-linking[2]
BHK cells stably expressing A52-taggedmutant A80C/R741C in the wild-type background were pre-treated for 5 min at 20 oCwith PBS containing 10 mM dithiothreitol (to reduce the disulfide bond) in thepresence of the following substrate or inhibitor: 500 nM tariquidar, 5 mMcyclosporine A, 10 mM vinblastine, 25 mM verapamil, 25 mM Taxol, 25 mMrhodamine B, 25 mM Hoechst 33342, 25 mM ketoconazole, 10 mM reserpine, 10 mM cis-flupentixol,10 mM trans-flupentixol or no drug substrate/ inhibitor. Cells were then washedfour times with PBS without dithiothreitol but containing the same substrate orinhibitor and then treated with or without 0.1 mM copper phenanthroline in thepresence of the same substrate or inhibitor for 3 min at 20oC. Thecells were then washed three times with PBS. Whole cell SDS (no reducing agent)extracts were then subjected to immunoblot analysis with monoclonal antibodyA52.
-
动物实验
MC26 Murine Colon Carcinoma Model[3]
The in vivo efficacy of XR9576 wasevaluated using the intrinsically resistant MC26 colon carcinoma tumors thatexhibited low levels of P-gp-mediated drug resistance. MC26 tumor slurry wasimplanted s.c. in BALB/c mice (day 0). The animals were then randomized, 24 hlater, into groups of 15–18 and treated once with various regimens. XR9576 orvehicle was administered either i.v. via a lateral tail vein or p.o. withdoxorubicin (5 mg/kg) or vehicle i.v. The modulator was administered eitheri.v. at 2–4 mg/kg (10 ml/kg) at the same time as doxorubicin or p.o. at 2–8mg/kg (10 ml/kg) 1 h before the cytotoxic drug. GG918 was administered p.o. 1 hbefore doxorubicin. All of the animals were weighed twice weekly. The animals werekilled by cervical dislocation on day 14, and the tumors were excised andweighed. The data were analyzed by Student’s t test.
HumanCarcinoma Xenografts
The efficacy of XR9576 was also evaluated usingMDR human carcinoma xenografts. Studies in parental (A2780) and resistant(2780AD) human ovarian carcinoma xenografts were performed. Briefly, A2780 and 2780ADcells grown in vitro were harvested by exposure to trypsin-EDTA, washed threetimes in PBS, and implanted s.c. in female nude mice (2 x 106cells in0.1 ml). When the tumors had reached a mean diameter of 0.5–1.0 cm, the animalswere randomized into groups of 6 and treated with various regimens on days 0(start of treatment), 2, and 4. XR9576 or vehicle was administered i.v 1 hbefore or p.o. 2 h before paclitaxel (15 mg/ml; formulated in 5% cremophor ELand 5% ethanol in (5% w/v) dextrose) or vehicle i.v. Administration of all ofthe i.v. compounds was via a lateral tail vein. The efficacy of GG918 was alsoevaluated after i.v. administration. Tumor volume and body weights wererecorded, and an ANOVA model was used to assess significance between treatmentgroups.
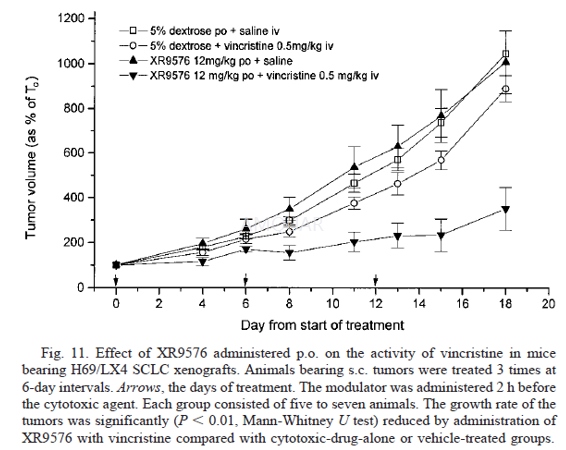
The ability of XR9576 to potentiate theantitumor activity of etoposide and vincristine was evaluated using the H69/LX4SCLC xenografts. The xenografts were established in female nude mice by s.c.implantation of 7–8 x 106 cells resuspended in PBS (0.1 ml) afterharvesting from in vitro culture. When the tumors had reached a mean diameterof about 0.6 cm, the animals were randomized into groups and treated on days 0,5, and 10 with XR9576 (i.v. or p.o.) and etoposide (30 mg/kg) or vincristine(0.5 mg/kg) i.v. The modulator and the cytotoxic drug were mixed togetherimmediately prior to i.v. administration by the caudal vein. Control animalswere treated with vehicle. Tumor volumes and body weights were recorded.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Weidner LD, Fung KL, Kannan P, et al. Tariquidar Is an Inhibitor and Not a Substrate of Human and Mouse P-glycoprotein. Drug Metab Dispos. 2016;44(2):275-282.
[2] Loo TW, Clarke DM. Tariquidar inhibits P-glycoprotein drug efflux but activates ATPase activity by blocking transition to an open conformation. Biochem Pharmacol. 2014;92(4):558-566.
[3] Mistry P SA, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61(2):749-758.
分子式
C38H38N4O6 |
分子量
646.73 |
CAS号
206873-63-4 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
49 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
30 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00069160 | Lung Neoplasms|Ovarian Neoplasms|Cervix Neoplasms|Renal Neoplasms | Drug: docetaxel|Drug: tariquidar|Other: 99mTc-sestamibi imaging | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 2 | 2003-09-01 | 2012-09-12 |
| NCT00071058 | Adrenal Cortex Neoplasms | Drug: XR9576 (Tariquidar) | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 2 | 2003-10-01 | 2012-09-12 |
| NCT00011414 | Wilms' Tumor|Sarcoma|Adenaocortical Carcinoma|Refractory Cancer|Coldrhood Cancer | Drug: Tariquidar | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 | 2001-02-15 | 2017-01-24 |
| NCT00082368 | Cancer | Drug: Tariquidar|Drug: Tc-94m Sestamibi | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 2 | 2004-05-01 | 2015-05-07 |
| NCT00001944 | Breast Cancer|Cancer|Lung Cancer|Ovarian Cancer | Drug: Vinorelbine|Drug: XR9576 | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 | 1999-12-01 | 2008-03-03 |
| NCT00042315 | Stage IIIb Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer | Procedure: Chemotherapy|Drug: tariquidar + vinorelbine|Drug: placebo + vinorelbine | QLT Inc. | Phase 3 | 2002-06-01 | 2012-05-22 |
| NCT00042302 | Stage IIIb Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer | Procedure: Chemotherapy|Drug: tariquidar + paclitaxel/carboplatin|Drug: placebo + paclitaxel/carboplatin | QLT Inc. | Phase 3 | 2002-06-01 | 2012-05-22 |
| NCT00048633 | Breast Neoplasms | Drug: Chemotherapy | QLT Inc. | Phase 2 | 2001-11-01 | 2012-05-22 |
| NCT01386476 | Drug Resistance | | National Institute of Mental Health (NIMH)|National Institutes of Health Clinical Center (CC) | | 2011-06-15 | 2017-01-24 |
| NCT01547754 | HIV-Associated Cognitive Motor Complex | | National Institute of Mental Health (NIMH)|National Institutes of Health Clinical Center (CC) | | 2012-01-09 | 2017-01-24 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们