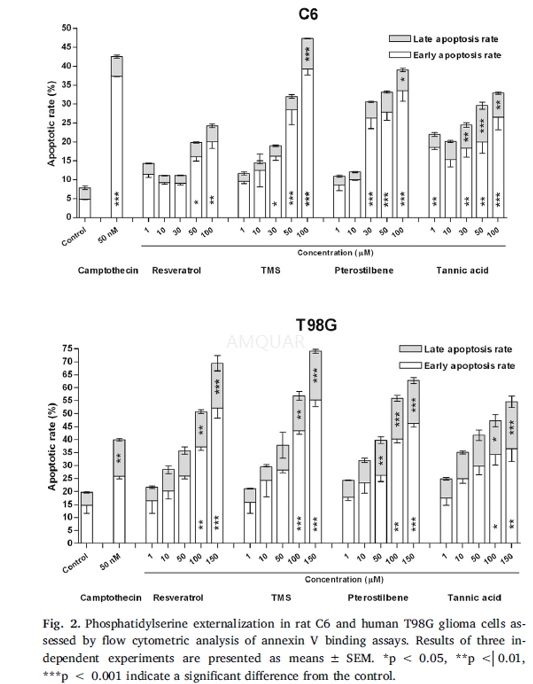
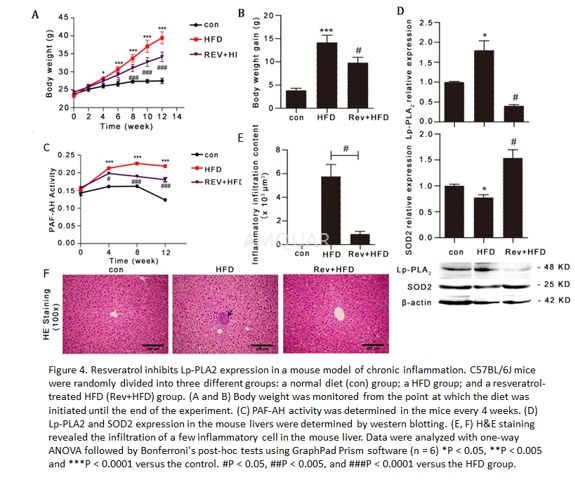
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01677611 | Type 2 Diabetes | Drug: Trans-resveratrol extract from Polygonum Cuspidatum|Drug: Placebo | Khoo Teck Puat Hospital|National Medical Research Council (NMRC), Singapore | Phase 1 | 2008-12-01 | 2012-08-31 |
| NCT02565979 | Pre-diabetes | Dietary Supplement: resveratrol|Dietary Supplement: placebo | Maastricht University Medical Center|Diabetes Fonds|DSM Nutritional Products, Inc. | | 2016-04-01 | 2016-08-17 |
| NCT01782911 | Androgen Profile|Inflammatory Markers|IVF Outcome | Dietary Supplement: Resveratrol|Dietary Supplement: Placebo pills | Instituto Valenciano de Infertilidad, IVI VALENCIA | Phase 3 | 2013-02-01 | 2013-01-31 |
| NCT01038089 | Type 2 Diabetes Mellitus | Dietary Supplement: Resveratrol | Boston University|DSM Nutritional Products, Inc. | | 2010-01-01 | 2010-12-17 |
| NCT00256334 | Colon Cancer|Cancer | Drug: Resveratrol | University of California, Irvine|University of California, Los Angeles | Phase 1 | 2005-07-01 | 2014-06-18 |
| NCT02245932 | Pulmonary Disease, Chronic Obstructive | Dietary Supplement: Resveratrol|Dietary Supplement: Placebo | Maastricht University Medical Center|The Netherlands Asthma Foundation|DSM Nutritional Products, Inc. | Phase 3 | 2015-01-01 | 2016-09-12 |
| NCT01476592 | Neuroendocrine Tumor | Dietary Supplement: Resveratrol | University of Wisconsin, Madison | | 2011-12-01 | 2016-06-15 |
| NCT01364961 | Dyslipidemia | Dietary Supplement: Resveratrol capsules | Maastricht University Medical Center|DSM Nutritional Products, Inc. | | 2011-01-01 | 2013-11-12 |
| NCT01339884 | Friedreich Ataxia | Drug: Resveratrol | Murdoch Childrens Research Institute|Friedreich's Ataxia Research Alliance | Phase 1|Phase 2 | 2011-04-01 | 2014-01-19 |
| NCT01638780 | Type 2 Diabetes | Dietary Supplement: placebo|Dietary Supplement: resveratrol | Maastricht University Medical Center|DSM Nutritional Products, Inc. | | 2012-05-01 | 2014-09-03 |
| NCT02247596 | Obesity|Diabetes | Drug: Resveratrol|Drug: Placebo | Khoo Teck Puat Hospital|National Medical Research Council (NMRC), Singapore | Phase 2 | 2009-07-01 | 2014-09-19 |
| NCT02129595 | Pre-diabetes | Dietary Supplement: placebo|Dietary Supplement: resveratrol | Maastricht University Medical Center|DSM Nutritional Products, Inc.|Diabetes Fonds | | 2014-04-01 | 2016-08-17 |
| NCT01010009 | Cognitive and Cerebral Blood Flow Effects of Resveratrol | Dietary Supplement: Trans- resveratrol|Other: Placebo (silica) | Northumbria University | | 2008-06-01 | 2012-05-17 |
| NCT01914081 | Non-ischemic Cardiomyopathy | Other: Resveratrol|Other: Placebo | St. Boniface General Hospital Research Centre|Canadian Centre for Agri-Food Research in Health and Medicine|Agriculture and Agri-Food Canada|Manitoba Medical Service Foundation | Phase 3 | 2015-01-01 | 2016-04-19 |
| NCT01451918 | Dyslipidaemia|Insulin Resistance | Drug: Resveratrol | University Health Network, Toronto|Canadian Institutes of Health Research (CIHR) | Phase 2 | 2011-10-01 | 2014-05-05 |
| NCT02549924 | Type 2 Diabetes Mellitus | Drug: Resveratrol|Drug: Placebo | University of Guadalajara | Phase 2 | 2015-09-01 | 2015-11-30 |
| NCT02621554 | Healthy | Dietary Supplement: resveratrol supplementation|Dietary Supplement: Placebo | Max Planck Institute for Human Cognitive and Brain Sciences|University of Leipzig|Evolva SA | | 2016-04-01 | 2017-02-06 |
| NCT01375959 | Impaired Glucose Tolerance | Dietary Supplement: resveratrol|Drug: Placebo | Albert Einstein College of Medicine, Inc.|American Diabetes Association | | 2011-04-01 | 2011-06-16 |
| NCT01412645 | Obesity|Inflammation|Insulin Sensitivity|Osteoporosis | Dietary Supplement: Resveratrol | University of Aarhus|The Ministry of Science, Technology and Innovation, Denmark|Central Denmark Region | | 2011-08-01 | 2013-12-12 |
| NCT02475564 | Endometriosis | Drug: Placebo|Drug: Resveratrol | Hospital de Clinicas de Porto Alegre | Phase 4 | 2015-06-01 | 2016-08-22 |
| NCT02433925 | Chronic Renal Insufficiency | Dietary Supplement: Resveratrol|Dietary Supplement: Placebo | Universidade Federal Fluminense | Phase 3 | 2013-01-01 | 2015-04-29 |