-
生物活性
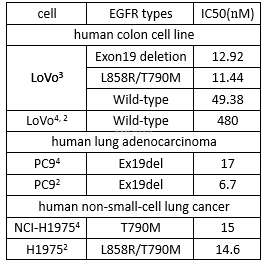
AZD9291 is an oral effective and irreversible third-generation EGFR tyrosine kinase inhibitor approved for patients with EGFR T790Mmutation-positive non-small cell lung cancer (NSCLC). AZD-9291 is a potent and selective mutated forms EGFR inhibitor (Exon 19 deletion EGFR IC50=12.92 nM, L858R/T790M EGFR IC50= 11.44 nM, wild type EGFR IC50= 493.8 nM). AZD9291 is highly active in preclinical models and is well tolerated in animal models. It inhibits both activating and resistant EGFR mutations while sparing the normal form of EGFR that is present in normal skin and gut cells, thereby reducing the side effects encountered with currently available medicines.
AZD9291 stimulates Vi-sensitive ABCB1 ATPase activitywith an EC50 of ~80nM.[1]
AZD9291 inhibits hERG with an IC50 of 16.2μM.[2]
In vitro potency against pEGFR of AZD9291
TheABCB1 transport function inhibition of AZD9291[1]

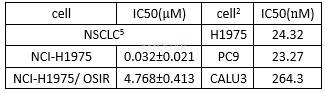
Cytotoxicityof AZD9291[1]

Anti-proliferation of AZD9291
-
体外研究
-
体内研究
1% DMSO+30% PEG 300+dd H2O
-
激酶实验
-
细胞实验
Reagents[6]
Osimertinib (OSI) and rociletinib weredissolved in dimethyl sulfoxide (DMSO) at a concentration of 20mM, stored at−20°C
Celllines and culture
NCI-H1975, HCC827, A549, and BEL-7402 cellswere cultured in a RPMI 1640 medium. A2780 cells were cultured in a DMEMmedium. HCT116 cells were cultured in a McCoy's 5A Medium. All cultured mediumswere supplemented with 10% (v/v) FBS and 1% (v/v) antibiotics (100 units/mLpenicillin and 100μg/mL streptomycin). Cells were grown in a 5% CO2 incubator at 37 °C.
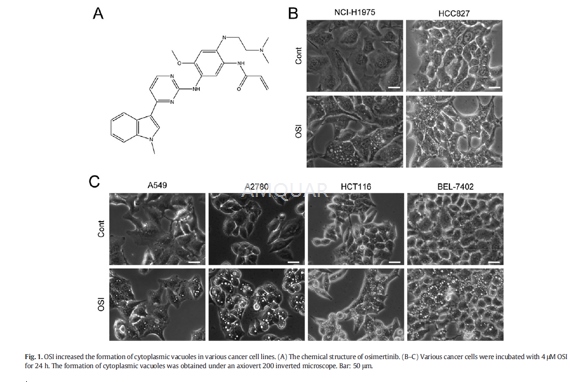
Observationof cytoplasmic vacuoles
NCI-H1975 cells (2 × 105cells/well),HCC827 cells (3 × 105cells/well), A549 cells (2 × 105cells/well),A2780 cells (2.5 × 105cells/well), HCT116 cells (2 × 105cells/well),and BEL-7402 cells (2 × 105cells/well) were plated into the 6-wellplate. After adhesion, cells were treated with 4μM OSI for 24h with or withoutpretreatment with different antioxidants. The formation of cytoplasmic vacuoleswas then observed with an axiovert 200 inverted microscope.
-
动物实验
Xenograft studies[4]
NCI-H1975 and A431 cells for in vivoimplant were cultured in DMEM supplemented with 10% v/v FCS and 1% v/vL-glutamine. PC9 cells were cultured in RPMI1640 supplemented with10%v/v FCS,1%v/v L-glutamine, and10% v/v M1 supplement. All cell lines were then culturedin a humidified incubator with 7.5% CO2 at 37oC. Cellswere detached using 0.05% trypsin and resuspended for implant in serum-freemedia. Cells (5 x 106) were implanted subcutaneously in a totalvolume of 0.1 mL per mouse for PC9 and NCI-H1975, and 1 x 107 cellsimplanted subcutaneously for A431. Both PC9 and A431 cells were implanted in50%Matrigel. PC9 xenografts were established in female severe combined immunecompromised (CB17 scid) mice, and NCIH1975 and A431 were established in femaleathymic (nu/nu: Alpk) mice. All mice were greater than 6 weeks old at the timeof cell implant. Tumor growth was monitored twice weekly by bilateral calipermeasurements, tumor volume calculated, and mice randomized into vehicle ortreatment groups with approximate mean start size of 0.2 to 0.4 cm3.Randomization for animal studies is based on initial tumor volumes to ensureequal distribution across groups. A power analysis is performed, in which groupsizes are calculated to enable statistically robust detection of tumor growthinhibition. Mice were dosed once daily by oral gavage for duration of thetreatment period. Tumor growth inhibition from the start of treatment wasassessed by comparison of the mean change in geometric tumor volume for thecontrol and treated groups. Statistical significance was evaluated using a one-tailedStudent t test.
For pharmacodynamic studies, mice wererandomized at a mean tumor volume of approximately 0.5 to 0.8 cm3 using the same randomization criteria as the tumor growth inhibition studies.Mice were then treated orally with either vehicle or osimertinib. Blood andtumor were taken at specific time points after dosing. Terminal blood sampleswere taken by cardiac puncture, centrifuged at 13,000 rpm, at 4C for 3 to 4minutes. Plasma was removed and stored at -80 oC.Tumors were snap frozen in liquid nitrogen, followed by storage at -80 oC.Tumors were homogenized by FastPrep technology in a Tris-based cell lysisbuffer containing phosphatase and protease inhibitors. Detection of EGFR andphosphorylated EGFR was performed using R&D Systems ELISA Kits (DYC1854 andDYC1095, respectively). Data are expressed as percentage inhibition ofphosphorylated: total protein compared with vehicle control group.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Hsiao SH, Lu YJ, Li YQ, Huang YH, Hsieh CH, Wu CP. Osimertinib (AZD9291) Attenuates the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCB1 in Vitro. Mol Pharm. 2016;13(6):2117-2125.
[2] Finlay MR, Anderton M, Ashton S, et al. Discovery of a potent and selective EGFR inhibitor (AZD9291) of both sensitizing and T790M resistance mutations that spares the wild type form of the receptor. J Med Chem. 2014;57(20):8249-8267.
[3] Sam M, Raymond, Verschoyle, Richard, Andrew, Vasantha, Krishna, Reddy, C., Andiappan, Heather, Marie, Inventor. 2-(2, 4, 5-Substituted-anilino) pyrimidine derivatives as EGFR modulators useful for treating cancer. US patent WO2013014448A12013.
[more]
分子式
C28H33N7O2 |
分子量
499.61 |
CAS号
1421373-65-0 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mg/mL |
Water
<1 mg/mL |
Ethanol
50 mg/mL |
体内溶解度
30 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02771314 | Non Small Cell Lung Cancer | Drug: AZD9291 | Hellenic Oncology Research Group | Phase 2 | 2016-06-01 | 2016-10-03 |
| NCT02736513 | Lung Cancer | Drug: AZD9291 | Rabin Medical Center | Phase 2 | 2016-05-01 | 2016-06-19 |
| NCT02972333 | EGFR-TKI Resistant Mutation|Nonsmall Cell Lung Cancer|AZD9291|Brain Metastases | Drug: AZD9291 80mg oral each day|Radiation: Radiation therapy | Shandong Cancer Hospital and Institute|AstraZeneca | Phase 3 | 2016-12-01 | 2016-11-20 |
| NCT02511106 | Stage IB-IIIA Non-small Cell Lung Carcinoma | Drug: AZD9291 80 mg/40 mg|Drug: Placebo AZD9291 80 mg/40 mg | AstraZeneca|Parexel | Phase 3 | 2015-10-01 | 2017-02-28 |
| NCT02157883 | Advanced Non Small Cell Lung Cancer|Advanced (Inoperable) Non Small Cell Lung Cancer | Procedure: Pharmacokinetic sampling|Drug: AZD9291|Drug: Itraconazole | AstraZeneca | Phase 1 | 2014-11-01 | 2017-01-09 |
| NCT02824952 | Lung Cancer | Drug: Tagrisso | Rabin Medical Center|AstraZeneca | Phase 2 | 2016-12-01 | 2016-11-17 |
| NCT02841579 | Non-Small Cell Lung Cancer | Drug: Osimertinib | MedSIR|AstraZeneca | Phase 2 | 2016-08-01 | 2017-01-03 |
| NCT02811354 | Carcinoma, Non-Small-Cell Lung | Drug: AZD9291 | National University Hospital, Singapore|AstraZeneca|Singapore Clinical Research Institute | Phase 2 | 2016-06-01 | 2016-06-21 |
| NCT02096679 | Healthy Volunteers | Drug: AZD9291 | AstraZeneca | Phase 1 | 2014-05-01 | 2015-10-01 |
| NCT02151981 | Anticancer Treatment | Drug: Chemotherapy|Drug: Cross-over to AZD9291 | AstraZeneca | Phase 3 | 2014-08-01 | 2017-01-23 |
| NCT02197247 | Non Small Cell Lung Cancer | Procedure: Pharmacokinetic sampling - AZD9291|Drug: Rifampicin|Drug: AZD9291 tablet dosing|Procedure: Pharmacokinetic sampling - rifampicin|Procedure: Pharmacokinetic sampling - AZ5140 and AZ7550 | AstraZeneca | Phase 1 | 2014-12-01 | 2017-02-03 |
| NCT02491944 | Oncology | Drug: AZD9291|Drug: [14C]AZD9291 | AstraZeneca | Phase 1 | 2015-07-01 | 2016-08-19 |
| NCT02224053 | Healthy Volunteer | Procedure: Pharmacokinetic sampling - AZD9291|Drug: AZD9291 tablet dosing|Drug: Omeprazole tablet dosing|Procedure: Pharmacokinetic sampling - AZ5140 and AZ7550 | AstraZeneca | Phase 1 | 2014-09-01 | 2016-05-23 |
| NCT02474355 | Lung Cancer | Procedure: T790M+ Testing|Procedure: Baseline Visit Blood & Urine Testing|Procedure: Baseline ECG|Procedure: Visual Slit-Lamp Testing|Drug: AZD9291 Dosing | AstraZeneca|Parexel | Phase 3 | 2015-09-01 | 2017-02-17 |
| NCT02442349 | Non-Small Cell Lung Cancer | Drug: AZD9291 | AstraZeneca | Phase 2 | 2015-06-01 | 2016-09-13 |
| NCT02094261 | Non Small Cell Lung Cancer | Drug: AZD9291 | AstraZeneca | Phase 2 | 2014-05-01 | 2016-11-21 |
| NCT01802632 | Advanced Non Small Cell Lung Cancer|Advanced (Inoperable) Non Small Cell Lung Cancer | Drug: AZD9291 | AstraZeneca | Phase 1|Phase 2 | 2013-03-01 | 2016-11-21 |
| NCT02163733 | Advanced Non Small Cell Lung Cancer|Advanced (Inoperable) Non Small Cell Lung Cancer | Drug: AZD9291 tablets|Procedure: Pharmacokinetic sampling - AZD9291|Other: Dietary Fasted|Other: Dietary High Fat|Procedure: Pharmacokinetic sampling - AZ5140 and AZ7550 | AstraZeneca | Phase 1 | 2014-11-01 | 2017-02-03 |
| NCT02317016 | Non Small Cell Lung Cancer | Procedure: Pharmacokinetic sampling - AZD9291|Drug: AZD9291 tablet dosing|Drug: Rosuvastatin|Procedure: Pharmacokinetic sampling - rosuvastatin|Procedure: Pharmacokinetic sampling - AZ5140 and AZ7550 | AstraZeneca | Phase 1 | 2015-03-01 | 2017-02-03 |
| NCT02161770 | Solid Tumours | Drug: AZD9291 tablet dosing|Procedure: Pharmacokinetic sampling - AZD9291|Procedure: Pharmacokinetic sampling - AZ5140 and AZ7550 | AstraZeneca | Phase 1 | 2014-12-22 | 2017-03-21 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们