-
生物活性
Bupivacaine hydrochloride,the hydrochloride form of bupivacaine, an amide local anesthetic, is employed innerve block, epidural, and intrathecal anesthesia and is often administered tocontrol pain before, during and after spinal surgery. Bupivacaine HCl is Na+ channel blocker, local anesthetic. Median doses of bupivacaine (in milligrams per kilogram) producing asystole in protocol 1 were for 17.7 for saline, 27.6 for 10% Intralipid, 49.7 for 20% Intralipid, and 82.0 for 30% Intralipid (P < 0.001 for differences between all groups). Differences in mean +/- SE concentrations of bupivacaine in plasma (in micrograms per milliliter) were significant (P < 0.05) for the difference between saline (93.3 +/- 7.6) and 30% Intralipid (212 +/- 45).
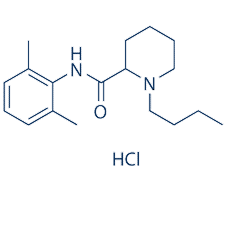
sodiumchannels effect of bupivacaine in adrenal chromaffin cells[1]
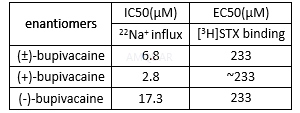
cAMPproduction inhibition of bupivacaine[2]

Celldestruction of bupivacaine in myocytes[3]
Bupivacaine induces a tonic block of Na+ currents in ND7/23 cells with an IC50 value of 178 ± 8μM.[4]
Bupivacaine inhibits nerve membrane with an IC50 of 200 nM.[5]
Bupivacaineinduced Schwann cell death with an LD50 of 476μM.[6]
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Cell culture[7]
HEK293 cells were grown on glass coverslipsin Dulbecco's modified Eagle medium (DMEM) containing 3.7 g/l NaHCO3 in addition to 2 mM L-glutamine, penicillin/streptomycin (10,000 E/10,000mg/ml) and 10% serum. Cells were kept at 5% CO2 at 37 °C. Cells wereused from passages 123–128, 3–6 days after trypsinization.
Patch-clampstudies
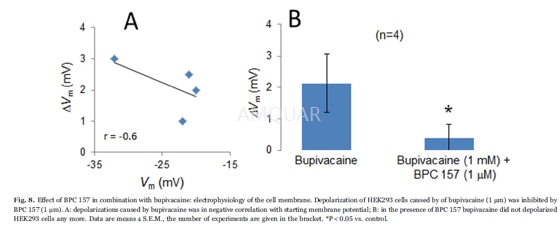
Coverslips with HEK293 cells were mountedat the bottom of a perfusion chamber on an inverted microscope. Membranevoltages (Vm) of HEK293 cells were measured using the slow-whole-cellpatch-clamp technique. A Ringers-type solution containing 145mM NaCl, 1.6mM K2HPO4,0.4mM KH2PO4, 5mM D-glucose, 1mM MgCl2 and1.3mM calcium gluconate (pH 7.4) was utilized. Bupivacaine (1mM) and BPC 157(1μm) were dissolved in this solution. That bupivacaine concentration of 1mMwas used because it was determined to be sufficient to allow binding to hERGpotassium channels.
All of the experiments were performed at 37°C with a bath perfusion rate of 10 ml/min. Patch-clamp pipettes were filledwith 95mM potassium gluconate, 30mM KCl, 4.8mM Na2HPO4,1.2mM NaH2PO4, 5mM D-glucose, 1.3mM calcium gluconate,1.03mM MgCl2 and 1mM ATP (pH 7.2). To this solution, 160μm nystatin wasadded to permeabilize the membrane under the pipette. The pipette resistancewas 5–10 MΩ. Vm was measured with a patchclamp amplifier and recordedcontinuously on a pen recorder.
-
动物实验
Animals[8]
The pregnant female Sprague-Dawley rats wereused. The rats were housed in a room on a 12 h light/dark cycle with freeaccess to water. The animals were housed individually in separate cages andmonitored closely for the day of birth, which was considered as postnatal day 0(P0). The rat pups (male and female) were kept in cages in a room on a 12 h light/darkcycle with free access to water with their littermates till P5. Neonataloverfeeding was done to induce obesity by reducing litter size to 3 pups perlitter (small litter, SL) on P6, while normal litters (NL) were culled to10pups per litter. The pups of SL were provided access to high fat diet from P6till P21. High fat diet was prepared with butter, milk powder, wheat flour andsugar in equal proportions each. The rats were fed with high fat diet at 4g/day along with standard rat chow. The animals of both SL (n = 36) andNL (n = 36) were monitored carefully. On P14, the rats were groupedseparately for experiments. The NL control pups (NLC) received no anesthesiaand were fed on normal standard diet and SL control pups (SLC) received no anesthesiabut were fed on high fat diet. The treatment groups NL and SL pups were inducedwith intrathecal bupivacaine (NLB and SLB) and ropivacaine (NLR and SLR) onP14. On P14, the body weights of NL pups were 23---28 g and that of SL pups werebetween 26 and 34 g.
Intrathecalinjections of bupivacaine and ropivacaine
With the animals in a prone position, thespinal solutions were injected intrathecally at the L4-L5 or L5-L6 level using a100μL syringe. Intrathecal placement of the needle tip was confirmed byobservation of a tail flick. A constant concentration of bupivacaine wasinjecting varying volumes scaled to the rat pup’s body weight at a dose of 5.0 mg·kg−1b.wt.Ropivacaine (7.5mg/kg b.wt.) was administered.
Behavioralassessments for sensory and motor blockade
The P14 rats underwent baseline measurementof hind paw thermal withdrawal latencies immediately before spinal injection.Blockade of thermal nociception was assessed using a modified hot plate test. Hindpaws were exposed in sequence (left then right) to a hot plate (model 39D hotplate analgesia meter) at 52oC for P14. The time (thermal withdrawallatency) until the rats lifted their paws was measured with a stopwatch. After12 s, the tested paw was removed by the experimenter to avoid injury to theanimal or the development of hyperalgesia. This test was repeated three times(with a 10s pause between tests) for each rat at every time point. Thermalwithdrawal latencies were measured every 10 min for at least 40 min after theintrathecal injection.
Blockade of mechanical nociception wasassessed by hind paw withdrawal using von Frey filaments. The von Frey filamentsapply logarithmically increasing pressure. Pups were lightly restrained on aflat surface and well calibrated von Frey hairs device that deliver increasingmechanical stimuli were applied to the dorsal surface of the hind paw of thepups, five times with one second intervals. The number of evoked withdrawalresponses to each stimulus of increasing intensity was recorded until a given stimulusevoked five responses or until a suprathreshold cut-off pressure was reached. Mechanicalwithdrawal thresholds were recorded at baseline and every 10 min for at least40 min after min after the intrathecal injection and until fullrecovery was observed. In both the thermal and mechanical withdrawal tests,animals were observed for the possibility of exhibiting motor blockade withoutsensory blockade based on absence of lower limb movement accompanied byvocalization or signs of upper body escape responses. This was not observed forany animal.
Motor performance of the lower extremitieswas assessed by a qualitative score. For each leg, if there was no spontaneousor evoked movement, the contribution to the score was zero. If there waspartial movement, the contribution was one; and if there was normal movement,the contribution to the score was two. Thus, in summing the values for bothlegs, the score could range from zero (complete blockade) to four (normal).
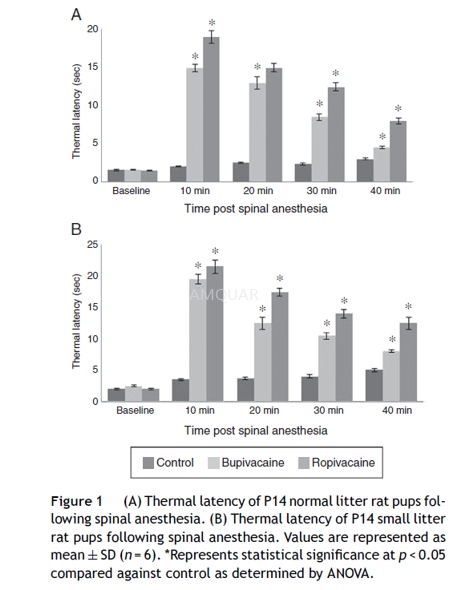
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Shiraishi S, Yokoo H, Yanagita T, et al. Differential effects of bupivacaine enantiomers, ropivacaine and lidocaine on up-regulation of cell surface voltage-dependent sodium channels in adrenal chromaffin cells. Brain Research. 2003;966(2):175-184.
[2] Butterworth JF 4th BR, Leith JP, Prielipp RC, Cole LR. Bupivacaine inhibits cyclic-3',5'-adenosine monophosphate production. A possible contributing factor to cardiovascular toxicity. Anesthesiology. 1993;79(1):88-95.
[3] Hofmann P, Metterlein T, Bollwein G, et al. The myotoxic effect of bupivacaine and ropivacaine on myotubes in primary mouse cell culture and an immortalized cell line. Anesth Analg. 2013;117(3):634-640.
[more]
分子式
C18H29ClN2O |
分子量
324.89 |
CAS号
18010-40-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
65 mg/mL |
Water
20 mg/mL |
Ethanol
65 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02255500 | Pain | Drug: Bupivacaine FNB|Drug: EXPAREL Infiltration | Pacira Pharmaceuticals, Inc | Phase 4 | 2014-09-01 | 2015-03-18 |
| NCT02662036 | Mammaplasty | Drug: Bupivicaine HCL|Drug: Liposomal bupivacaine | Washington University School of Medicine | Phase 2 | 2016-02-29 | 2017-02-08 |
| NCT02284386 | Pain | Drug: Bupivacaine SNB|Drug: EXPAREL Infiltration | Pacira Pharmaceuticals, Inc | Phase 4 | 2014-12-01 | 2016-05-20 |
| NCT02805504 | Urinary Tract Diseases | Drug: Exparel|Drug: Marcaine | Loma Linda University | Phase 4 | 2016-06-01 | 2016-12-15 |
| NCT02737813 | Cardiac Output|Hypotension|Anesthesia, Spinal | Drug: Isobaric marcaine|Drug: Hyperbaric marcaine | Mahidol University | Phase 4 | 2016-03-01 | 2016-04-09 |
| NCT02352922 | Pain, Postoperative|Surgical Procedure, Unspecified | Drug: Liposomal Bupivacaine|Drug: Bupivacaine HCl | Florida Hospital | Phase 4 | 2015-07-01 | 2016-08-22 |
| NCT02274870 | Post-operative Pain | Drug: Liposome Bupivacaine|Drug: Bupivacaine HCl | Northwell Health | Phase 4 | 2014-11-01 | 2017-01-05 |
| NCT01861665 | Post-operative Pain | Drug: Marcaine- 0.25%|Drug: Marcaine 0.25%. | Weill Medical College of Cornell University | | 2010-06-01 | 2014-01-06 |
| NCT01636869 | Serum Bupivacaine Level|Periarticular Block|Total Knee Arthroplasty | Drug: Bupivacaine | Mahidol University | Phase 4 | 2012-07-01 | 2014-07-25 |
| NCT01494259 | Pain | Drug: Bupivacaine|Drug: Saline | Johns Hopkins University | Phase 4 | 2016-01-01 | 2015-10-09 |
| NCT01977352 | Shoulder Pain|Rotator Cuff Tear | Drug: Liposomal bupivacaine|Drug: Bupivacaine 0.25% | St. Luke's-Roosevelt Hospital Center | Phase 4 | 2014-01-01 | 2015-06-02 |
| NCT02426164 | Pain, Postoperative|Arthroplasty, Replacement, Knee|Osteoarthritis | Drug: Liposomal bupivacaine|Drug: bupivacaine HCl, morphine, epinephrine, methylprednisolone | Miller Orthopedic Specialists|Creighton University Medical Center|CHI Health Mercy Hospital | Phase 4 | 2015-06-01 | 2016-03-18 |
| NCT00993746 | Infraclavicular Brachial Plexus Block | Drug: Bupivacaine plus lidocaine|Drug: Bupivacaine 30 ml | Mahidol University | Phase 4 | 2009-10-01 | 2010-05-14 |
| NCT01616108 | Strabismus|Esotropia|Exotropia|Graves Disease|Nystagmus | Drug: Bupivacaine | Smith-Kettlewell Eye Research Institute|Eidactics|Sutter Health|Strabismus Research Foundation | Phase 2|Phase 3 | 2012-04-01 | 2016-10-13 |
| NCT02349542 | Arthroplasty, Replacement, Knee|Pain | Drug: Liposomal bupivacaine | OrthoCarolina Research Institute, Inc. | Phase 4 | 2015-01-01 | 2017-02-08 |
| NCT01303731 | Cesarean Section|Anesthesia, Spinal|Local Anaesthetics Causing Adverse Effects in Therapeutic Use | Drug: Bupivacaine and Fentanyl|Drug: Bupivacaine | Bnai Zion Medical Center | Phase 4 | 2011-02-01 | 2011-02-23 |
| NCT02015182 | Transversus Abdominis Plane Block | Other: Blood sample for bupivicaine pharmacokinetics | The Hospital for Sick Children | | 2011-09-01 | 2016-04-14 |
| NCT02499159 | Pain | Drug: Liposomal Bupivicaine|Drug: 0.25% standard bupivicaine | Inova Health Care Services|Mednax National Medical Group | Phase 4 | 2014-12-01 | 2017-03-07 |
| NCT02121119 | Hallux Valgus | Drug: Lidocaine|Drug: Bupivacaine | Pontificia Universidad Catolica de Chile | Phase 4 | 2013-09-01 | 2016-05-22 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们