-
生物活性
Canertinib (CI-1033), an ATP-competitive irreversiblepan-ErbB inhibitor, shows potent effects against several cancers, includingbreast cancer, NSCLC and advanced ovarian cancer. Canertinib is a potent and selective inhibitor of tyrosine residue phosphorylation of EGFR (IC50 = 1.5nM) which blocks signal transgression and ceasing angiogenisis. Additionally, Canertinib's action in inhibiting EGFR increases apoptosis in cancerous cell lines in a titratable fashion.
Kinase activities of canertinib

Autophosphorylation inhibition of canertinib

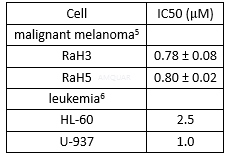
Cytotoxicity of canertinib

Anti-proliferation of canertinib
-
体外研究
-
体内研究
30% propylene glycol, 5% Tween 80, 65% D5W(5%葡萄糖水溶液)
-
激酶实验
Tyrosine Kinase Assays[1]
EGFR tyrosine kinase was purified. Enzyme assaysfor IC50 [app] determinations were performed in 96-well filter plates. Thetotal volume was 0.1mL containing 20mM Hepes, pH 7.4, 50mM sodium vanadate,40mM magnesium chloride, 10μM adenosine triphosphate (ATP) containing 0.5mCi of [32P]ATP,20 mg of polyglutamic acid/tyrosine, 10ng of EGFR tyrosine kinase, andappropriate dilutions of inhibitor. All components except the ATP were added tothe well and the plate was incubated with shaking for 10 min at25 °C. Thereaction was started by adding [32P] ATP, and the plate wasincubated at 25 °C for 10 min. The reaction was terminated by addition of 0.1mL of 20% trichloroacetic acid (TCA). The plate was kept at 4 °C for at least15 min to allow the substrate to precipitate. The wells was then washed five timeswith 0.2 mL of 10% TCA and 32P incorporation determined with aWallac beta plate counter.
-
细胞实验
Cells and culture conditions[7]
Jurkat cells were cultured at 37 ℃ with 5% CO2 in RPMI 1640 supplemented with 10% (v/v)heat-inactivated fetal calf serum, 2mM L-glutamine and penicillin (50units/ ml),and streptomycin (50μg/ml). The cells were subcultivated three times per week and kept ata density of 0.1–1 x 106 cells/ml. The cell concentration in all experimentswas ~0.5 x 106cells/ml, except for the proliferation assay where 0.3x 106cells/ml was used.
Chemicals
The pan-ErbB receptor tyrosine kinaseinhibitor, canertinib was used. A working solution of 400μMwas prepared in growth medium and used within 30 min of preparation. Gefitinib,the cytotoxic monoclonal FasL/anti-Fas IgM (CH-11), inhibitors of caspase-8(Z-IETD-FMK), caspase-9 (Z-LEHDFMK), caspase-10 (Z-AEVD-FMK), Cysteine proteaseinhibitor E64d and camptothecin were prepared. All compounds were dissolved inDMSO and the resulting concentration of DMSO during experiments was less than 0.1%in the cell cultures.
Cellcycle analysis
Exponentially growing Jurkat cells wereincubated with or without 2μM canertinib for 24 h. The cell cycle distribution was determined.The DNA content was analyzed by flow cytometry using a FACSCalibur™. Thefractions of G1-, S-, and G2/M-phase cells were determined using Mod-Fit Lt3.0.
Flowcytometry
The number of apoptotic Jurkat cells wasanalyzed by flow cytometry using the Annexin V-PE Apoptosis Detection Kit I. Toensure measurement of early apoptosis, the cells were counterstained with avital dye, 7-aminoactinomycin D (7-AAD), which has strong affinity for DNA.Annexin V-PE and 7-AAD fluorescence intensities were measured within 1h by flowcytometry analysis. Annexin V-positive and 7-AAD-negative cells were consideredearly apoptotic cells. Out of a total 15,000 events the cells (~14,000 cells gatedper analysis) were gated in a FSC/SSC scattergram to exclude cell debris andsmall particles before performing analysis using the CellQuest Pro software.The results are expressed as mean fraction of labelled cells.

-
动物实验
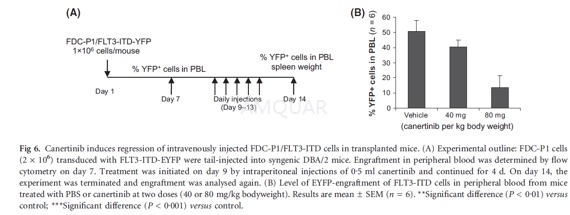
In vivo experiments[8]
FDC-P1 cells (2 x 106)transduced with retrovirus expressing FLT3-ITD and enhanced yellow fluorescenceprotein (EYFP) were injected into the tail vein of syngenic DBA/2 mice in 0.2ml phosphate-buffered saline (PBS) per mouse. Seven days later, the level ofengraftment was analysed by flow cytometry on peripheral blood samplescollected by lateral-tail vein bleeding. Treatment was initiated on day 9 byintraperitoneal injections of 0.5 ml of canertinib (40 or 80 mg/kg/d) in PBS andcontinued for four consecutive days (day 10–13). On day 14, the experiment wasterminated and a new blood sample was taken to determine the engraftment aftertreatment. Mice were then sacrificed and spleen weights were recorded.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Smaill JB RG, Loo JA, Greis KD, Chan OH, Reyner EL, Lipka E, Showalter HD, Vincent PW, Elliott WL, Denny WA. Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido[3,2-d]pyrimidine-6-acrylamides bearing additional solubilizing function. J Med Chem. 2000;43(7):1380-1397.
[2] Slichenmyer WJ, Elliott WL, Fry DW. CI-1033, a pan-erbB tyrosine kinase inhibitor. Seminars in Oncology. 2001;28(5N):80-85.
[3] Prasasya RD, Vang KZ, Kreeger PK. A multivariate model of ErbB network composition predicts ovarian cancer cell response to canertinib. Biotechnol Bioeng. 2012;109(1):213-224.
[more]
分子式
C24H25ClFN5O3 |
分子量
485.94 |
CAS号
267243-28-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
2 mg/mL |
Water
<1 mg/mL |
Ethanol
9 mg/mL |
体内溶解度
约10 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00050830 | Lung Neoplasms | Drug: CI 1033 | Pfizer | Phase 2 | 2003-01-01 | 2006-11-06 |
| NCT00051051 | Breast Neoplasms | Drug: CI-1033 | Pfizer | Phase 2 | 2002-12-01 | 2007-05-03 |
| NCT00174356 | Carcinoma, Non-Small Cell Lung | Drug: CI 1033|Drug: PACLITAXEL|Drug: CARBOPLATIN | Pfizer | Phase 1 | 2002-12-01 | 2006-11-06 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们























