-
生物活性
Tofacitinib (CP-690550, Tasocitinib), a specificorally active reversible competitive JAK3 inhibitor with immunosuppressiveproperties, is the first approved drug for treatment of rheumatoid arthritis. Tofacitinib is an inhibitor of JAK2. Tofacitinib is a novel inhibitor of JAK3 with IC50 of 1 nM.
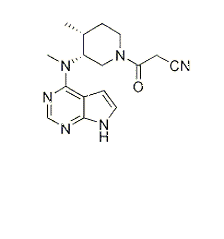
IL-15-inducedCD69 expression inhibition of CP-690550[1]
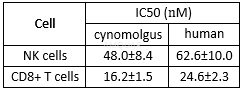
Mixed lymphocyte reaction inhibition of CP-690550
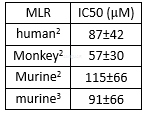
Enzyme activities of CP-690550[2]
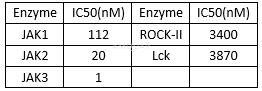
Anti-proliferationof CP-690550[2]
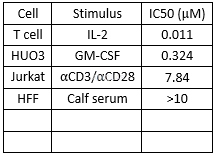
Antiviralpotency of tofacitinib[4]
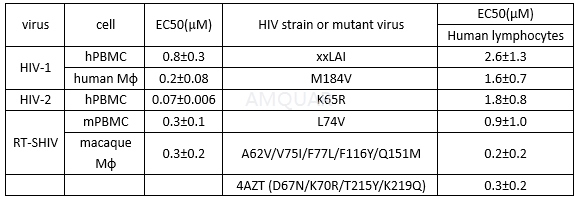
hPBMC, human peripheral blood mononuclearcells; mPBMC, macaque peripheral blood mononuclear cells; Mφ, macrophages.
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% propylene glycol
-
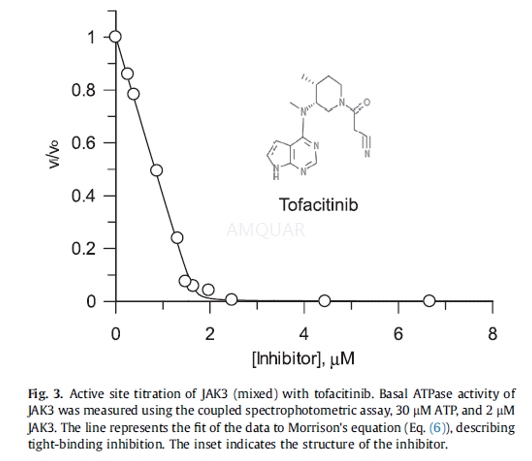
激酶实验
Kinase activity assay[5]
The kinase activity of JAK3 was measuredusing a radioactive, end-point assay. All concentrations are final in thereaction mixture. The reaction was carried out in a volume of 40μLcontaining standard assay buffer, composed of 50mM Hepes pH 7.2, 10mM MgCl2,1mM DTT, and 0.5-1mg/mL BSA, 1600-50,000cpm/pmol [γ-33P]ATP, 0.5-30μM unlabeled ATP, 20-750μM J1L peptide substrate, andtypically 0.1nM JAK3. Reactions were incubated at 23 ℃ and terminated by the addition of EDTA to a final concentration of 50mM. Aportion of terminated reactions (typically 25μL) wastransferred to 150-200μL of streptavidin beads suspended in 1xPBS containing 50mM EDTA in aMultiScreen 96-well filter plate. In order to ensure the binding capacity ofthe beads was not exceeded, the concentration (v/v) of beads was varieddepending on the concentration of the peptide in the reaction. Followingincubation of terminated reactions with streptavidin beads at 23 ℃ for 30 min, the beads were washed with 200μL of first 2MNaCl (4times), then 2M NaCl plus 1% phosphoric acid (4 times) and finally water(two times). Washed plates were allowed to dry either at 23oCovernight or in a 60oC oven for 1h. Plates were counted in aPerkinElmer TopCount microplate scintillation counter after at least 1h ofincubation in the presence of scintillation cocktail. The concentration of phosphopeptideproduct was calculated using the specific activity of ATP. Initial rateconditions resulting in linear formation of thephosphopeptide product withtime, a linear change in reaction rate in response to varying JAK3concentrations, and less than 10-15% consumption of the limiting substrate weredetermined from time course experiments.
-
细胞实验
B-Cell Culture[6]
B lymphocytes (5 x 103) wereincubated in wells of a 96-well plate. The medium was Iscove modified Dulbeccomedium (IMDM) supplemented with 10% fetal calf serum, 0.05mmol/L 2-mercaptoethanol,and ITS (insulin, transferrin, and selenium, 1,000-fold diluted). B cells wereco-cultured with irradiated (75Gy) CD40 ligand-transfected fibroblasts(L-CD40L) as feeder cells and a cocktail of B-cell-activating cytokine added onday 0 to enhance B-cell survival at 37oC in a 5% CO2 humidified incubator. Cytokine cocktail included interleukin (IL) 2 at 100 U/mL,IL-10 at 25ng/mL, and IL-21 at 100ng/mL
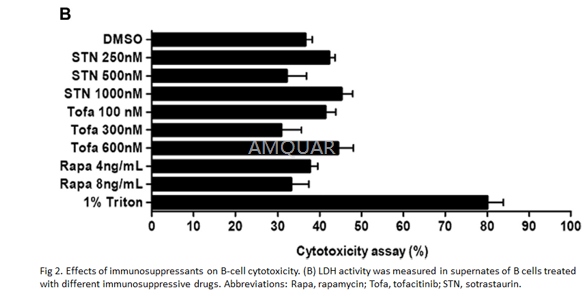
ImmunosuppressiveDrugs
Tofacitinib was used at 100, 300, and600nmol/L, and STN was used at 250, 500, and 1,000nmol/L. Both drugs werediluted in dimethyl sulfoxide (DMSO). Rapamycin was dissolved in methanol andused at concentrations of 4ng/mL and 8ng/mL. Working solutions for all of theimmunosuppressive drugs were made in culture medium.
CytotoxicityAssay
To test whether the immunosuppressive drugswere toxic to B-cell culture, a lactate dehydrogenase (LDH) activity assay wasperformed according to the manufacturer’s instructions. Briefly, 104 B cells/well were cultured in 96-well round-bottom plates in the presence ofthe immunosuppressive drugs. The culture was performed in IMDM supplemented with1% human serum, to avoid the interference with LDH, and0.05mmol/L 2-mercaptoethanol.After 16 hours of incubation, cells were spun and 100 mL cell-free supernateswere incubated with 100μL of the kit’s substrate for 30 minutes. Plates were read on the absorbancemicroplate reader with filters 490 nm to 620 nm, and results were expressed asUI/μL.
ApoptosisAssay
The percentage of apoptotic cells afterculture was measured with the use of propidium iodide (PI) and annexin V-fluoresceinisothiocyanate(FITC). In brief, B cells were cultured at 2 X 105 cells/well with 5 X 104 L-CD40L cells (as feeders) in the presenceof immunosuppressive drugs at different concentrations. After 2 days, cellswere harvested and annexin V- FITC and PI were added and incubated for 10minutes on ice. Cells were acquired with the use of a FACS Canto II cytometerand analyzed with the use of BD FACSDiva (v6.1.2) software, and someexperiments were repeated and analyzed with the use of Flowjo software v 10.
-
动物实验
Adjuvant-induced arthritis (AIA)[7]
Female Lewis rats were purchased. Arthritiswas induced in 7–8-week-old rats by a subcutaneous injection of 0.1μl ofcomplete freund's adjuvant emulsifiedwith nonviable 10mg/ml Mycobacterium tuberculosisH37RA (Difco) injected intradermal at the base of the tail.
Treatmentgroups
After immunization, rats were randomlyassigned to 3 experimental groups (n = 10/group): Tofacitinib (6.2 mg/kg b.w.),methotrexate (MTX) (0.3 mg/kg b.w.), and a control untreated group. Tofacitinib(CP-690,550) was dissolved in 2% Tween 80, 0.5% Methylcellulose in water andwas stirred and vortex vigorously to create a very fine aqueous suspension.Tofacitinib was administered by oral gavage (0.2-ml volume) once a day,initiating at day 7 post disease induction continued through the end of theexperiment (day 29 post disease induction). MTX (ABITREXATE, 25 mg/ml ABIC) wasdiluted in saline and was administered in oral dose twice aweek (total of 7times administrations). Two independent experiments were performed. Naïveagematched rats served as a control group for measurement of blood serum CRPlevels and mRNA expression levels in the spleen.
Assessmentof arthritis
Rats were examined for disease progressionbeginning at day 0 for baseline evaluation and between days 10 to 30 postdisease induction were monitored twice a week for two clinical parameters:arthritis score and changes in paw diameter. Measurement of paw swelling was measuredby the same observer, who was blinded to the treatment the animal received. Thepaw diameter was measured in millimeters with digital micro-caliper, measuringof the thickness of the fore and hind limbs, respectively. Paw swelling waspresented as the mean thickness of the fore and hind limbs (in mm), change inpaw volume was presented as the mean ± SEM.
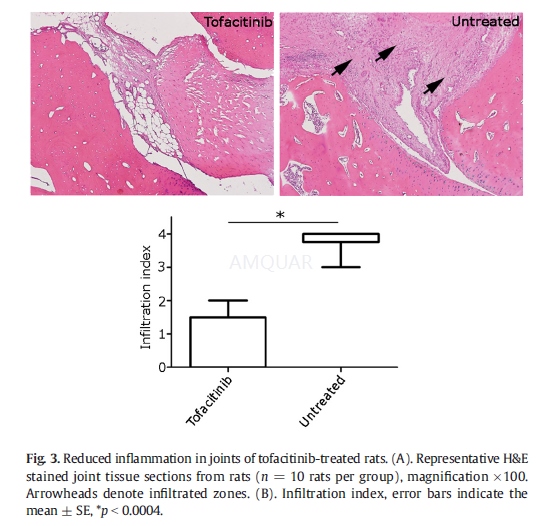
Histologicand immunohistochemistry assessment
At the end of the experiments the rats weresacrificed and hind paws were dissected for histopathological examination.Isolated tissues specimens were decalcified fixed in formalin and processed forroutine paraffin block preparation. Multiple representative sections (6μmthick) were cut and stained with hematoxylin and eosin and visualized underlight microscopy. The extent of joint inflammation and destruction of bone andcartilagewas determined using a semi-quantitative inflammation score. Theseveritywas scored on a scale of 0–3: grade 0, no signs of inflammation; grade1, mild inflammation with hyperplasia of the synovial lining and minorcartilage damage; grades 2–3, increasing degrees of inflammatory cellinfiltrate and destruction of bone and cartilage.
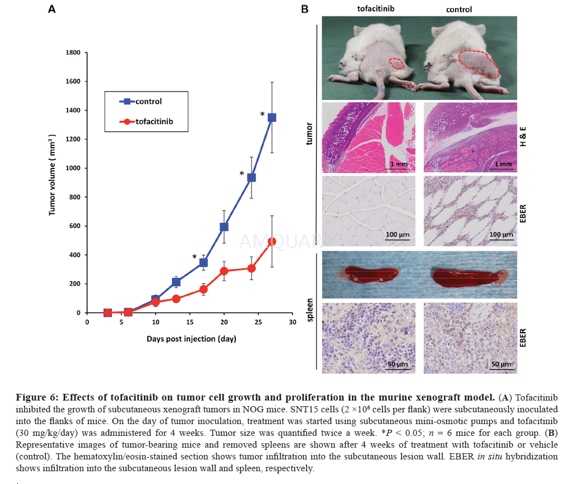
Xenograft model[8]
NOG mice were maintained under specificpathogen-free conditions. SNT15 cells (2 × 106 cells per flank) weresuspended in a volume of 100μL and subcutaneously inoculated into the rightflanks of mice. Mini-osmotic pumps (ALZET) were implanted into the left flankson the same day. Tofacitinib was dissolved in a sterile solution of 50% DMSO,10% PEG and 40% saline solution and was delivered at a flow rate of 0.5μL/h forfour weeks. Mice were randomized to drug –related or control groups. Tumor sizewas quantified with calipers twice a week, and peripheral blood was obtainedevery week.
Immunohistochemistry
All mice were humanely killed and the body tissuesincluding tumors, brain, heart, kidney, lung and spleen were removed.Formaldehyde-fixed, paraffin-embedded tissues were cut into serial sections about3-μm thick. Sections were then stained with hematoxylin and eosin and werestained for EBER by in situ hybridization using the EBER PNA Probe/FITC and theVECTASTAIN Elite ABC kit, according to the manufacturers’ protocols.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Conklyn M. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. Journal of Leukocyte Biology. 2004;76(6):1248-1255.
[2] Changelian PS FM, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des
[3] Kudlacz E, Perry B, Sawyer P, et al. The Novel JAK-3 Inhibitor CP-690550 Is a Potent Immunosuppressive Agent in Various Murine Models. American Journal of Transplantation. 2004;4(1):51-57.
[more]
分子式
C16H20N6O |
分子量
312.37 |
CAS号
477600-75-2 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
56 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约12 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT03002649 | Dermatomyositis | Drug: Tofacitinib | Johns Hopkins University|Pfizer | Phase 1 | 2017-01-01 | 2017-01-25 |
| NCT02281552 | Rheumatoid Arthritis | Drug: Tofacitinib|Drug: Tofacitinib | Pfizer | Phase 3 | 2014-11-01 | 2017-01-17 |
| NCT03011281 | Rheumatoid Arthritis | Drug: Tofacitinib | Hanyang University | | 2016-11-01 | 2017-01-04 |
| NCT02299297 | Alopecia Areata | Drug: Tofacitinib | Julian M. Mackay-Wiggan|Locks of Love|Columbia University | Phase 2 | 2015-01-01 | 2017-02-14 |
| NCT02592434 | Juvenile Idiopathic Arthritis | Drug: CP-690,550 (tofacitinib)|Other: placebo | Pfizer | Phase 3 | 2016-06-10 | 2017-03-15 |
| NCT02566967 | Rheumatoid Arthritis | Drug: tofacitinib | Norman B. Gaylis, MD|Arthritis & Rheumatic Disease Specialties Research | Phase 3 | 2015-05-01 | 2017-01-23 |
| NCT02812342 | Alopecia Areata|Alopecia Totalis|Alopecia Universalis | Drug: Tofacitinib ointment | Yale University | Phase 2 | 2016-09-01 | 2016-12-15 |
| NCT02535689 | Systemic Lupus Erythematosus | Drug: Tofacitinib|Drug: Placebo | National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|National Institutes of Health Clinical Center (CC) | Phase 1 | 2015-08-25 | 2017-01-24 |
| NCT02487433 | Healthy | Drug: Tofacitinib MR 22 mg (Fed)|Drug: Tofacitinib MR 22 mg (Fasted) | Pfizer | Phase 1 | 2015-06-01 | 2015-08-17 |
| NCT02084875 | Healthy | Drug: tofacitinib modified-release (MR) formulation|Drug: tofacitinib modified-release (MR) formulation | Pfizer | Phase 1 | 2014-04-01 | 2014-07-09 |
| NCT01500551 | Juvenile Idiopathic Arthritis | Drug: Tofacitinib|Drug: Tofacitinib | Pfizer | Phase 3 | 2013-03-18 | 2017-03-15 |
| NCT02321930 | Rheumatoid Arthritis | Drug: tofacitinib 5mg po bid | University of California, Los Angeles | Phase 4 | 2016-01-01 | 2016-08-26 |
| NCT01976364 | Arthritis, Psoriatic | Drug: Tofacitinib|Drug: Tofacitinib | Pfizer | Phase 3 | 2014-02-17 | 2017-03-21 |
| NCT02831855 | Rheumatoid Arthritis | Drug: CP-690,550|Drug: Methotrexate|Drug: Placebo | Pfizer | Phase 4 | 2016-09-01 | 2017-03-10 |
| NCT01731327 | Healthy | Drug: tofacitinib modified-release (MR) formulation|Drug: tofacitinib modified-release (MR) formulation | Pfizer | Phase 1 | 2012-11-01 | 2012-12-20 |
| NCT02147587 | Rheumatoid Arthritis | Drug: Tofacitinib|Drug: Placebo | Pfizer | Phase 2 | 2014-06-01 | 2015-07-31 |
| NCT01164579 | Rheumatoid Arthritis | Drug: Tasocitinib plus Methotrexate|Drug: Tofacitinib plus placebo methotrexate|Drug: Placebo tofacitinib plus Methotrexate | Pfizer | Phase 2 | 2010-10-01 | 2015-04-21 |
| NCT01499004 | Healthy | Drug: tofacitinib (CP-690,550) modified-release formulation A|Drug: tofacitinib (CP-690,550) modified-release formulation B1|Drug: tofacitinib (CP-690,550) modified-release formulation A|Drug: tofacitinib (CP-690,550) modified-release formulation B1|Drug: tofacitinib (CP-690,550) modified-release formulation B2|Drug: tofacitinib (CP-690,550) immediate-release formulation | Pfizer | Phase 1 | 2011-11-01 | 2012-01-11 |
| NCT01599377 | Healthy | Drug: tofacitinib 10 mg|Drug: tofacitinib 5 mg | Pfizer | Phase 1 | 2012-05-01 | 2012-07-08 |
| NCT01202240 | Healthy | Drug: Tasocitinib (CP-690,55) plus Ketoconazole | Pfizer | Phase 1 | 2010-09-01 | 2011-02-15 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们