-
生物活性
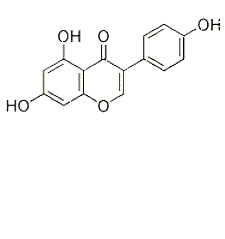
Genistein (NPI 031L), a specific inhibitor of tyrosine-specific proteinkinases, can induce apoptosis of various cancer cells, including leukemia, colorectal,hepatoma, prostate and breast cancer cells. Genistein is a highly specific inhibitor of protein tyrosine kinase (PTK). Genistein is an inhibitor of Glucosidase. Additionally, Genistein is a competitive inhibitor of ATP in other protein kinase reactions. Genistein inhibits tyrosine phosphorylation in isolated enzyme and receptor preparations and in whole cells including platelets, lymphocytes and a variety of cultured cells. It also inhibits EGF-stimulated phosphorylation in cultured cells as well as inhibition of Topo II (topoisomerase II). In cultured A431 epidermoid carcinoma cells, EGF-stimulated tyrosine phosphorylation was completely inhibited by genistein at 100 μg/ml. Inhibition is competitive with ATP and noncompetitive with substrate.
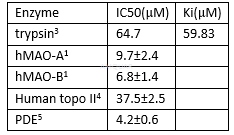
Genistein inhibits the H2O2 production byhMAOB (IC50 2.9 ± 0.2 𝜇M) and hMAO-A (IC50 28.9 ± 0.1 𝜇M). [1]
Genisteininhibits LPS-stimulated NO production with an IC50 of 69.4μM.[2]
Enzyme activity of genistein
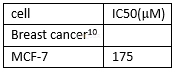
Anti-proliferativeeffects of genistein
Cytotoxic effect of genistein
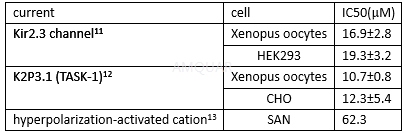
Currents inhibition of genistein
Anti-rotavirus effects of genistein[14]

-
体外研究
-
体内研究
-
激酶实验
Measurement of topo activity[4]
The catalytic activity of topo I wasdetermined by detecting supercoiled plasmid DNA (form I) in its nicked form(form II). The topo I reaction was performed in a 20μl reaction mixture thatcontained 10mM Tris-HCl (pH 7.9), pBR322 DNA (250ng), 1mM EDTA, 150mM NaCl,0.1% bovine serum albumin (BSA), 0.1mM spermidine, 5% glycerol, 2μl of one ofthe 6 test compounds 1-6 dissolved in DMSO, and 2 units of topo I.
The catalytic activity of topo II wasanalyzed in the same manner, except the reaction mixture contained 50mMTris-HCl (pH 8.0), 120mM KCl, 10mM MgCl2, 0.5mM ATP, 0.5mMdithiothreitol, supercoiled pBR322 DNA (250ng), and 2units of topo II. Thereaction mixtures were incubated at 37˚C for 30min, followed by digestion with1% sodium dodecyl sulfate (SDS) and 1mg/ml proteinase K. After digestion, 2μlloading buffer, consisting of 5% sarkosyl, 0.0025% bromophenol blue, and 25%glycerol, was added.
To study the binding of enzymes to DNAbased on mobility shifts, the same procedure was followed, but SDS denaturationand proteinase K digestion were omitted. The mixtures were subjected to 1%agarose gel electrophoresis in Tris/borate/EDTA buffer. Agarose gel was stainedwith ethidium bromide (EtBr) and the DNA band shifts from form I to form II bytopos I and II were detected using an enhanced chemiluminescence detectionsystem. Zero-D scan was used for densitometric quantitation.

-
细胞实验
Drug preparation[9]
Genistein was dissolved in dimethylsulfoxide (DMSO) and stored in small aliquots at -20oC.
Cellculture
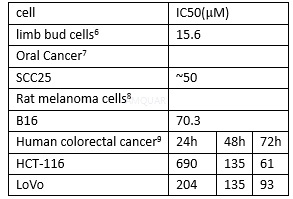
Human colorectal cancer cell lines, HCT-116and LoVo were maintained as a monolayer in DMEM supplemented with 10% fetal bovineserum (FBS) at 37oC in a humidified 5% CO2 incubator.
MTTassay
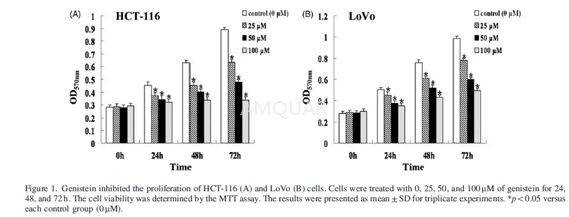
The effect of genistein on cell viabilitywas assessed by MTT assay. HCT-116 and LoVo cells were seeded in 96-well platesat a density of 4x103 cells per well and incubated for 24 h. Then,media were replaced with fresh media containing different concentrations (0,25, 50, and 100μM) of genistein and cultured for 24, 48, and 72 h. After treatment,20μl MTT (5mg/ml) was added to each well and further incubated for4 h.The supernatant was aspirated and replaced with 150μl/ well ofDMSO to dissolve the formazan salt formed. The optical density (OD) wasmeasured at 570nm using a microplate reader.
Flowcytometry
The apoptotic rates of the HCT-116 cellsand LoVo cells were determined using an annexin V-FITC apoptosis detection kit.In brief, cells were seeded in six-well plates overnight, then treated withvarious concentrations of genistein for 48 h, collected, and resuspended inbinding buffer. Annexin V-FITC and propidium iodide (PI) were added accordingto the manufacturer’s instructions. The analysis was performed with an FACSCalibur Flow Cytometer.
-
动物实验
Chemicals[15]
For the in vivo experiments, genistein wassolubilized in PEG-400 on the day of the experiments by 20 s of sonication.Genistein and the 0.1 ml PEG-400 vehicle were injected intraperitoneally.
Mousetreatment
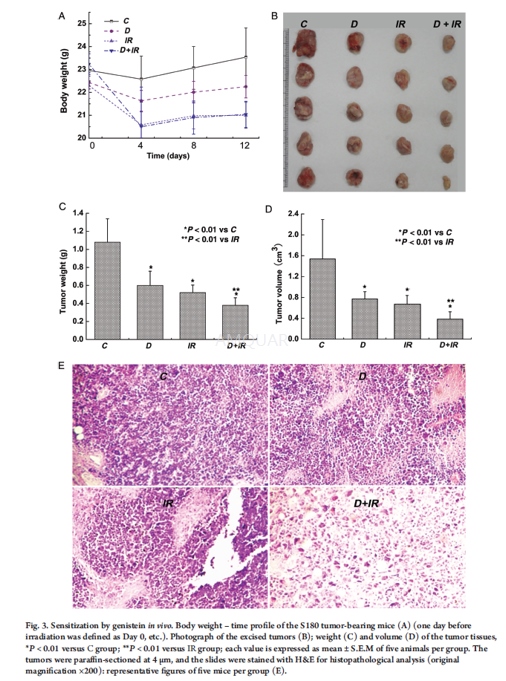
Eight-week-old female Balb/c mice (20 ± 2g) of Specific Pathogen-Free (SPF) grade were maintained on a 12-h light–darkcycle at temperature of 22 ± 1°C. During the experiments, mice were provided withsterilized food and water ad libitum. After being fed for one week, 20 micewere inoculated with S180 cells. All mice were subcutaneously implanted with 1× 106 ascites cells/mouse at the backside. Ten days afterinoculation, the animals were randomly divided into the following four groups(each group containing five mice). The first group, the control group (C), was treatedwith the vehicle; the second group, the drug group (D), was given anintraperitoneal injection of genistein (200mg/kg body weight). The third groupof mice, the irradiated group (IR), was administered an intraperitonealinjection of the vehicle and received fractionated whole-body irradiation. Thefourth group (D+IR) was administered an intraperitoneal injection of genistein(200mg/kg body weight), and 24 h later, the mice were given fractionatedwholebody exposure to X-rays in the same manner as the third group. The micewere sacrificed by cervical dislocation 24 h after the last irradiation. Tumortissues were immediately removed and frozen at –80°C for biochemicalexamination. For histological evaluation, fresh samples were initially fixed in4% formaldehyde–phosphate buffer solution.
Irradiation
After genistein pretreatment, cells andmice were irradiated with X-rays, which were generated with an X-ray machineoperated at 50 kVp. The dose rate was ∼0.5Gy/min. All irradiations were carried out at room temperature.S180 cells received single-fraction irradiation of 2 Gy X-rays, whereas5-fraction irradiations at 2Gy/fraction were given to each mouse once every twodays, i.e. equivalent to a 10Gy single dose.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Zarmouh NO, Messeha SS, Elshami FM, Soliman KF. Evaluation of the Isoflavone Genistein as Reversible Human Monoamine Oxidase-A and -B Inhibitor. Evid Based Complement Alternat Med. 2016;2016:1423052.
[2] Choi C CH, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFkappaB activation in RAW 264.7 macrophages. Biosci Biotechnol Biochem. 2003;67(9):1916-1922.
[3] Zeng HJ, Wang YP, Yang R, You J, Qu LB. Inhibitory effects of daidzein and genistein on trypsin: Insights from spectroscopic and molecular docking studies. Int J Biol Macromol. 2016;89:336-343.
[more]
分子式
C15H10O5 |
分子量
270.24 |
CAS号
446-72-0 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
<1 mg/mL |
Ethanol
8 mM |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01985763 | Colon Cancer|Rectal Cancer|Colorectal Cancer | Drug: Genistein | Sofya Pintova|DSM Nutritional Products, Inc.|Icahn School of Medicine at Mount Sinai | Phase 1|Phase 2 | 2013-11-01 | 2017-01-25 |
| NCT02499861 | Cancer | Drug: Decitabine and Genistein | St. Justine's Hospital | Phase 1|Phase 2 | 2015-07-01 | 2016-05-08 |
| NCT00882765 | Pancreatic Cancer | Dietary Supplement: genistein | Jonsson Comprehensive Cancer Center|National Cancer Institute (NCI) | Phase 2 | 2009-05-01 | 2015-10-28 |
| NCT02796794 | Sepsis | Dietary Supplement: Genistein|Other: enteral nutrition only | TC Erciyes University | Phase 4 | 2015-06-01 | 2016-06-07 |
| NCT00769990 | Breast Cancer|Kidney Cancer|Lung Cancer|Melanoma|Metastatic Cancer|Pain|Prostate Cancer | Dietary Supplement: genistein|Radiation: radiation therapy | Masonic Cancer Center, University of Minnesota | Phase 1|Phase 2 | 2008-09-01 | 2011-07-01 |
| NCT01126879 | Adenocarcinoma of the Prostate|Recurrent Prostate Cancer|Stage I Prostate Cancer|Stage II Prostate Cancer|Stage III Prostate Cancer | Dietary Supplement: genistein|Other: placebo|Procedure: therapeutic conventional surgery | Northwestern University|National Cancer Institute (NCI) | Phase 2 | 2010-05-01 | 2016-08-15 |
| NCT00541710 | Metabolic Syndrome | Dietary Supplement: genistein|Dietary Supplement: placebo | University of Messina|Ministry of Education, Universities and Research, Italy | Phase 2|Phase 3 | 2007-10-01 | 2012-09-13 |
| NCT02766478 | Prostate Cancer | Drug: Genistein|Drug: Placebo | Emory University | Phase 2 | 2017-05-01 | 2017-03-03 |
| NCT01489813 | Bladder Cancer | Drug: Genistein|Drug: Sugar pill | Emory University | Phase 2 | 2017-07-31 | 2017-01-18 |
| NCT02624388 | Lymphoma|Childhood Lymphoma|Solid Tumor|Childhood Solid Tumor|Neuroblastoma|Ewing Sarcoma|Hodgkin Lymphoma|Non-Hodgkin Lymphoma|Rhabdomyosarcoma|Soft Tissue Sarcoma|Medulloblastoma|Germ Cell Tumor|Wilms Tumor|Brain Neoplasms|Medulloblastoma, Childhood|Neuroectodermal Tumors, Primitive | Drug: Genistein|Drug: Placebo | University of Virginia | Phase 2 | 2016-08-01 | 2016-08-11 |
| NCT01325311 | Prostate Adenocarcinoma|Stage I Prostate Cancer|Stage IIA Prostate Cancer|Stage IIB Prostate Cancer | Drug: Cholecalciferol|Drug: Genistein|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Other: Placebo | National Cancer Institute (NCI) | Phase 2 | 2011-12-01 | 2016-06-20 |
| NCT00626769 | Menopause|Osteopenia | Dietary Supplement: aglycone genistein|Dietary Supplement: placebo | University of Messina|Primus Pharmaceuticals | Phase 2|Phase 3 | 2005-07-01 | 2009-05-18 |
| NCT00276835 | Kidney Cancer|Melanoma (Skin) | Biological: High-dose interleukin-2|Dietary Supplement: genistein | Northwestern University|National Cancer Institute (NCI) | Early Phase 1 | 2005-11-01 | 2015-04-08 |
| NCT00590538 | Cystic Fibrosis | Drug: Sodium 4-Phenylbutyrate|Drug: Genistein (Unconjugated Isoflavones 100)|Drug: Placebo | Children's Hospital of Philadelphia|Cystic Fibrosis Foundation Therapeutics | Phase 1|Phase 2 | 2003-02-01 | 2011-06-29 |
| NCT00016744 | Cystic Fibrosis | Drug: Sodium 4-Phenylbutyrate (4PBA)|Drug: Unconjugated Isoflavones 100 (PTI G-4660, 87% Genistein)|Drug: Placebo | Children's Hospital of Philadelphia|Cystic Fibrosis Foundation Therapeutics|National Center for Research Resources (NCRR) | Phase 1|Phase 2 | 2001-09-01 | 2009-01-08 |
| NCT01664650 | Metabolic Syndrome | Dietary Supplement: Genistein|Dietary Supplement: Placebo | University of Messina|Ministry of Education, Universities and Research, Italy | Phase 2|Phase 3 | 2008-09-01 | 2012-08-09 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们