-
生物活性
Plerixafor 8HCl (AMD3100 8HCl) is a octahydrochloride form of the bicyclammolecule AMD3100, a SDF-1/ CXCR4 signaling antagonist which was initially usedfor inhibition HIV virus entry, but now used as a hematopoietic stem cellmobilization agent.
Plerixafor is a selective bicyclam derivative which functions as a stem cell mobiliser via blocking the CXCR4 chemokine receptor. Highly selective CXCR4 chemokine receptor antagonist (IC50 values are 0.02 - 0.13 and > 25 μM for CXCR4 and all other chemokine receptors respectively). Studies suggest that Plerixafor causes the rapid movement of stem cells out of bone marrow via blocking the CXCR4 receptor. Since the CXCR4 receptor is responsible for regulating metastasis in chemoresistant melanoma cells, blocking this receptor can possibly reduce the metastasis of melanoma cells. In addition, studies indicate that Plerixafor can inhibit the replication of human immunodeficiency virus in vitro by blocking the entry of the virus into cells. Furthermore, Plerixafor can also reduce the number of human CD4+ T cells. Switches inflammatory responses from Th2 to Th1 type and reduces airway hyperresponsiveness in a mouse model of asthma. Potently inhibits HIV-1 and HIV-2 replication in vitro (EC50 = 4 - 35 nM) and mobilizes hematopoietic stem cells in vivo.
AMD3100inhibits CXCR4 (IC50=44 nM) [1]
AMD3100 inhibits CXCL12-mediated chemotaxis (IC50=5.7nM) [1]
AMD3100 inhibits SDF-1/CXCL12 ligand binding (IC50 = 651±37 nM) [2]
AMD3100inhibits SDF-1mediated GTP-binding (IC50 = 27.3 ± 2.2 nM), SDF-1 mediated calcium flux (IC50 =572 ± 190 nM), and SDF-1 stimulated chemotaxis (IC50= 51 ± 17 nM). [2]
-
体外研究
-
体内研究
Saline
-
激酶实验
Receptor binding assays[2]
For the competition binding studies againstCXCR4, a concentration range of AMD3100 was incubated for 3 h at4oCin binding buffer (PBS containing 5mM MgCl2, 1mM CaCl2,0.25% BSA, pH 7.4) with 5 x 105 CCRF–CEM cells and100pM 125I-SDF-1α (2200 Ci/mmol) in Milipore DuraporeTM filter plates. Unbound 125I-SDF-1α was removed by washing with cold 50mM HEPES, 0.5M NaCl pH 7.4. The competitionbinding assay against BLT1 was performed on membranes from CHO-S cellsexpressing recombinant BLT1. The membranes were prepared by mechanical celllysis followed by high speed centrifugation, re-suspended in50mm HEPES, 5mMMgCl2 buffer and flash frozen. The membrane preparation wasincubated with AMD3100 for 1 h at room temperature in an assay mixturecontaining 50mM Tris, pH 7.4, 10mM MgCl2, 10mM CaCl2, 4nMLTB4 mixed with1nM 3H-LTB4 (195.0Ci/mmol) and 8mg membrane. Theunbound 3H-LTB4 was separated by filtration on Millipore Type GF-C filterplates. The bound radioactivity was counted using a LKB Rackbeta 1209 Liquid ScintillationCounter.

-
细胞实验
Cell culture[3]
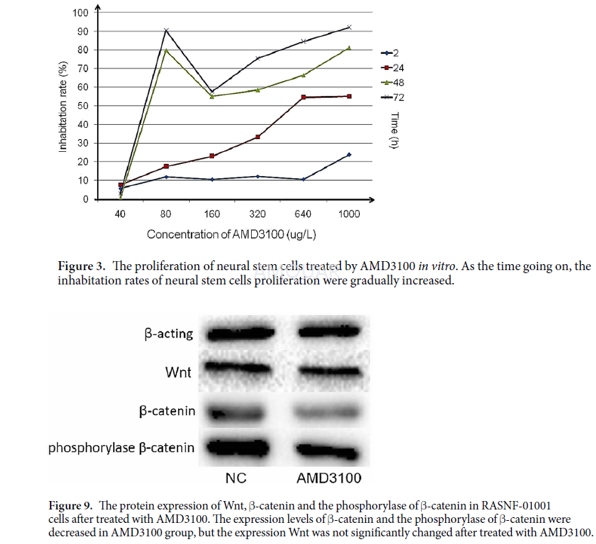
The Sprague-Dawley rat neural stem cells(NSCs) line of RASNF-01001 were cultured in RASNF-90011 medium at 37 °C in ahumidified atmosphere of 5% CO2. After the neural spheres formationand getting large, the passage was carried out.
Cellproliferation assay
The NSCs proliferation ability wasdetermined with the Cell Counting Kit-8 (CCK-8) assay . The Cells were culturedin 96-well tissue culture plates at a cell density of 1 × 104 cells/well. The plate was incubated for 24h at 37°C in a humidified atmosphereof 5% CO2. After added AMD3100 to the medium, the cells wereincubated for 24, 48 and 72h. Then, 10μL CCK-8 solution were added into eachwell and further cultured for 2h. The optical density (OD) was measured at 490nmwave length. The experiments were repeated three times over multiple days.
Westernblotting
Western blotting assay was used to detectthe protein expression of Nestin, β-Tubulin, Wnt1, β-Catenin and phosphorylaseof β-Catenin (Phospho-Tyr489). After treated with AMD3100, the cellular lysatesof RASNF-01001 were obtained using RIPA lysis buffer containing 6μg/ml PMSF.Total protein was extracted and the protein concentration was determined byBradford assay. Western blotting assay was carried out using antibodies againstNestin, β-tubulin, Wnt1, β-catenin and phosphorylase β-catenin(Phospho-Tyr489). The immune complexes were measured with pro-light HRP Kit.Expression of proteins was analyzed using ImageJ software. All the experimentswere repeated three times over multiple days.
Woundhealing assay
The ability of NSCs migration was assessedby wound healing assay. The cells were grown to confluence in 6-well tissueculture plate at a density of 5 × 106 cells/well after treated withAMD3100. The cells were then denuded by dragging a rubber policeman through thecenter of the plate. And the cells were incubated at 37 °C for 72h. ImagesJsoftware was used to measure the distance of the NSCs migration in the well at24, 48 and 72h, respectively. All the experiments were repeated three timesover multiple days.
-
动物实验
Animals[4]
Three-month-old male Sprague–Dawley ratswere obtained (n = 5 for rBMSCs harvest; n = 3 for normal bone harvest; n = 15for fracture model; n = 35 for DO model). All rats were housed in plastic cagesat 25 ± 1 °C and constant humidity and had free access to standard laboratorychow and sterile water.
RatFracture Model
Fifteen rats (belonging to the fracturegroup) were anesthetized using xylazine (4mg/kg) and ketamine (40mg/kg), administeredintraperitoneally. A mid-diaphysis transverse osteotomy was performed on theright tibia under sterile conditions. A monolateral external fixator/lengthenerwas assembled to fix two segments with four stainless steel pins, followed bythe sequential suture of the incision. Animals belonging to the fracture groupwere sacrificed at day 5, 10, 15, 29, and 43 (n = 3 at each time point) aftersurgery. At day 0, normal tibia segments from three rats belonging to thecontrol group were harvested.
RatDO Model
A total of 35 rats were operated undergeneral anesthesia and sterile conditions. The surgical procedure was the sameas previously described for the rats belonging to the fracture group. Followinga 5-day latency period, lengthening was initiated and maintained at a rate of0.25 mm/12 h for 10 days, and the total lengthening was 5 mm. After the lengtheningcompleted, the position of bone segments was maintained by the externalfixator/lengthener for another 4 weeks before termination. Fifteen ratsdesignated as DO group were used for comparison with the fracture group. Ratswere sacrificed at day 5, 10, 15, 29, and 43 (n = 3 at each time point) aftersurgery. The remaining twenty rats were randomly divided into two groups:phosphate-buffered saline (PBS) group (n = 10) and AMD3100 group (n = 10). Fromthe initiation of the lengthening period, animals were injected with either100μL of PBS or 100μL of AMD3100 solution (at a concentration of 400μM in PBS)into the distraction gap every 2 days until their termination. AMD3100 was usedat the concentration of 400μM in PBS, which was shown to inhibit SDF-1/Cxcr4signaling effectively, without causing toxicity. Calcein (10 mg/kg) and xylenolorange (30 mg/kg) were subcutaneously injected into the rats at day 16 and day40 following initial surgery, respectively. At termination, bilateral tibiaswere harvested by removing the fixators at day 43 after the surgery, forfurther analyses.
HistologicalAnalyses
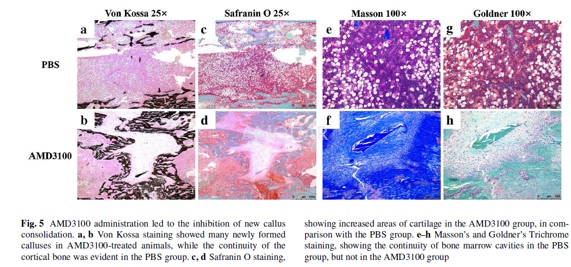
Following the animal termination, bonespecimens (n = 3 per group) were dehydrated in a graded series of alcohol andxylene and embedded in methyl methacrylate. Each sample was cut in half with aSP1600 saw microtome along the long axis of the tibia in the midsagittal plane,and 5- and 10-μm-thick sections were cut with an RM2155 hard tissue microtomealong the long bone axis. The 5-μm sections were stained with Von Kossa,Safranin O, Masson’s Trichrome, and Goldner’s Trichrome stains, in order toperform static histomorphometric analysis, while unstained 10-μm sections wereused for dynamic histomorphometric measurements, performed using a fluorescencemicroscope. Bone analysis software, OsteoMeasure, was used to analyze theobtained parameters, including the ratio of mineralized surface to bone surface(MS/BS), mineral apposition rate (MAR), bone formation rate per unit of bonesurface (BFR/ BS), bone formation rate per bone volume (BFR/BV), and boneformation rate per TV (BFR/TV).
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Zabel BA, Wang Y, Lewen S, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183(5):3204-3211.
[2] Fricker SP, Anastassov V, Cox J, et al. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72(5):588-596.
[more]
分子式
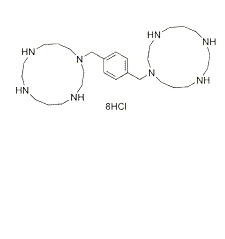
C28H54N8.8HCl |
分子量
794.47 |
CAS号
155148-31-5 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
<1 mg/mL |
Water
100 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约35 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01149863 | Autologous Transplantation | Drug: Plerixafor | Emory University|Genzyme, a Sanofi Company | Phase 2 | 2010-06-01 | 2013-11-19 |
| NCT01319864 | Relapsed/Refractory AML|Relapsed/Refractory ALL|Secondary AML/MDS|Acute Leukemia of Ambiguous Lineage|AML|ALL | Drug: Plerixafor Dose Escalation | Seattle Children's Hospital|Children's Healthcare of Atlanta|Pediatric Oncology Experimental Therapeutics Investigation Consortium | Phase 1 | 2011-03-01 | 2016-10-17 |
| NCT01042717 | Multiple Myeloma|Non-Hodgkins Lymphoma | Drug: Plerixafor | Shi, Patricia, M.D.|Genzyme, a Sanofi Company | | 2010-02-01 | 2011-09-26 |
| NCT01141543 | Acute Myeloid Leukemia | Drug: Plerixafor (mozobil) | University Health Network, Toronto|Princess Margaret Hospital, Canada | | 2010-05-01 | 2014-07-16 |
| NCT01455025 | Acute Myeloid Leukemia | Drug: Plerixafor granulocyte-colony stimulating factor | French Innovative Leukemia Organisation|Acute Leukemia French Association|Genzyme, a Sanofi Company | Phase 1 | 2012-01-01 | 2016-03-15 |
| NCT01280955 | Failure of Bone Marrow Graft | Drug: Plerixafor | Mitchell Horwitz, MD|Genzyme, a Sanofi Company|Duke University | Phase 1|Phase 2 | 2011-12-01 | 2017-02-06 |
| NCT01339572 | Non-Hodgkin's Lymphoma|Multiple Myeloma | Drug: Plerixafor|Drug: Filgrastim | University of Florida | Phase 4 | 2011-04-01 | 2016-10-25 |
| NCT00082329 | Healthy | Drug: AMD 3100 (Mozobil plerixafor) | Richard Childs, M.D.|National Heart, Lung, and Blood Institute (NHLBI)|National Institutes of Health Clinical Center (CC) | Phase 2 | 2004-05-01 | 2014-04-01 |
| NCT00694590 | Chronic Lymphocytic Leukemia (CLL)|Small Lymphocytic Lymphoma (SLL) | Drug: plerixafor | Genzyme, a Sanofi Company|Sanofi | Phase 1 | 2008-06-01 | 2015-03-19 |
| NCT00903968 | Multiple Myeloma | Drug: Plerixafor (AMD3100)|Drug: bortezomib | Dana-Farber Cancer Institute|Brigham and Women's Hospital|Genzyme, a Sanofi Company|Millennium Pharmaceuticals, Inc. | Phase 1|Phase 2 | 2009-05-01 | 2017-01-18 |
| NCT00445302 | Renal Impairment | Drug: plerixafor | Genzyme, a Sanofi Company|Sanofi | Phase 1 | 2006-01-01 | 2014-02-10 |
| NCT01068301 | Acute Lymphoblastic Leukemia|Acute Myeloid Leukemia|Chronic Myeloid Leukemia|Myelodysplastic Syndrome|Non-Hodgkin's Lymphoma | Drug: Plerixafor | St. Jude Children's Research Hospital|Genzyme, a Sanofi Company | Phase 1 | 2010-05-01 | 2013-12-31 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们