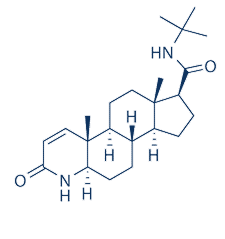
-
生物活性
Finasteride, a type II 5-α reductase inhibitor (5-ARI) that blocks the conversion of testosterone (T) intodihydrotestosterone (DHT), is used for the treatment of benign prostatichyperplasia and has been suggested to act as a chemopreventive agent forprostate cancer. Finasteride is an inhibitor of 5α-Reductase 1. Finasteride is a synthetic 4-azasteroid antiandrogen compound. This specifically inhibits 5α-Reductase 2, an intracellular enzyme that converts the androgen testosterone into 5α dihydrotestosterone (DHT). Finasteride inhibits 5α-reductase activity in epithelium for Ki of 10nM, significantly lower than in stroma (Ki = 33nM). Antiandrogen that inhibits type II 5α reductase (IC50 = 65 nM). Suppresses the conversion of testosterone to dihydrotestosterone. Reduces prostatic dihydrotestosterone levels and prostate size in vivo.
Finasterideinhibits echinoid 5α-reductase with IC50 of 2.7μM for both ovariesand testes.[1]
Finasterideinhibits 5α-reductase with an IC50 of approximately 25 nM for both fetalgenital tubercle and adult ventral prostate.[2]
5-α reductase inhibition of finasteride

-
体外研究
-
体内研究
-
激酶实验
Inhibition of 5α-reductase activity in vitro[1]
Reproductively mature echinoids were maintainedin aquaria (32 ppt salinity, 22°C) for 3 weeks and fed ad libitum a formulateddiet impregnated in 3% agar. These individuals were dissected and gonads wereexcised, blotted dry and weighed. Sex was determined by microscopic evaluationof gonadal smears. Ovaries (n‑3) and testes (n‑3) were suspended in artificialseawater (ASW) buffered with 20mM HEPES (32 ppt salinity, pH 7.4) (1:19 w:v)and homogenized using a Polytron@ Brinkman Homogenizer. Equal aliquots ofhomogenates of both ovaries and testes were each incubated in ASW buffered with20mM HEPES (32 ppt salinity, pH 7.4) containing 1mM NADPH, 1μCi/ml (37 kBq /ml) 3H-androstenedione (specific activity 338mCi /mg,12.5GBq/ mg), and concentrations of crystalline finasteride ranging from 0 to805μM suspended in 3% propylene glycol and buffer. Incubations wereterminated after 90min with 3volumes of methylene chloride (MC).Organic-soluble steroid metabolites were isolated and identified. Briefly,organic-soluble steroid metabolites were extracted in 3x 3 volumes of MC,concentrated under a stream of nitrogen gas, resolubilized in a small volume ofchloroform:methanol (9:1) and transferred to thin-layer chromatography (TLC)plates. The following chromatography solvent systems were used to isolatesteroid metabolites:
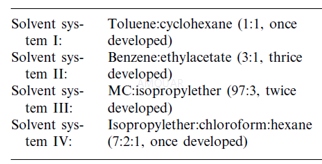
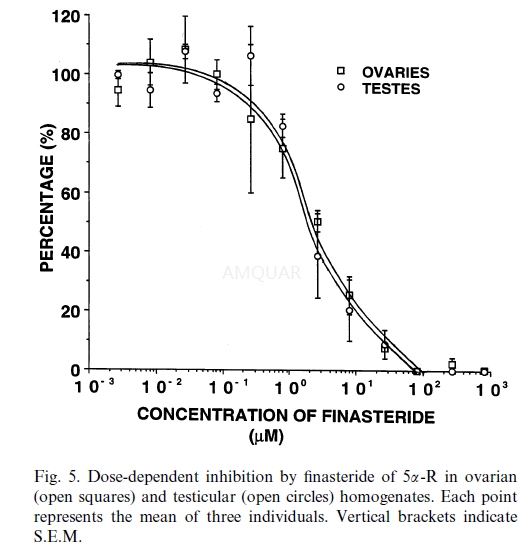
5αRs inhibition assay[4]
Cell pellets containing recombinant rattypes 1 or 2 5αR were resuspended in 1M sucrose, 1mM 3-[Nmorpholino] propanesulfonicacid (MOPS), pH7.2, 20mM potassium chloride, 1mM dithiothreitol (DTT), 1mMphenylmethyl sulfonyl fluoride and 5mM NADPH. The cells were broken by three freeze/thawcycles in a dry ice/methanol bath. The suspension was then sonicated for 4 x 30s intervals on ice, with 2min between sonications. The suspension was stored at-80°C.
The reaction mixture for the rat type 1 5αRcontained 33mM succinic acid, 44mM imidazole, 33mM diethanolamine (SID), pH6.5, 2μM [3H]-T, 1mM DTT and 0.5mM NADPH in a final volume of0.1ml. The assay was initiated by the addition of enzyme and incubated at 37°Cfor 20-30min. The assay mixture of the rat type 2 5αR containedsimilar components, but in this case the SID buffer was pH 5.5 and theconcentration of [3H]-T was 0.15pM. For both isozymes, the reactionwas quenched with cyclohexane:ethylacetate (70:30v/v) and T separated from DHTby normal phase HPLC.
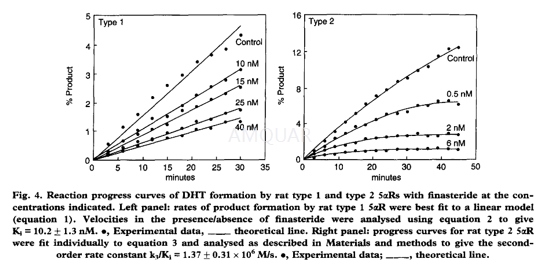
-
细胞实验
Cells and cell culture conditions[5]
Initiated benign prostate epithelial cells(BPH-1) were cultured in RPMI 1640 medium supplemented with 2 mmol/ LL-glutamine, 10% fetal bovine serum (FBS) (Gibco, Melbourne, Australia), and 1%penicillin-streptomycin at 37oC with 5% CO2. c-Jun+/+ andc-Jun−/− mouse embryonic fibroblasts were cultured in Dulbecco's modifiedEagle's medium (DMEM) supplemented with 2mmol/ L L-glutamine, 10% FBS, and 1%penicillin-streptomycin at 37oC with 5% CO2. Theco-culture experiments were performed. Briefly, BPH-1 cells were cultured in0.4μm pore size permeable membrane transwell inserts, and mouse fibroblastswere cultured in 6-well plates. When 50% confluent, BPH-1 cells were starved inFBS-free DMEM for 24 h, then moved to the 6-well plate with 80% confluentfibroblasts. The stromal-epithelial co-cultures were maintained in 1% FBS DMEMmedium containing 100μmol/ L finasteride previously prepared in DMSO, and themedium was replaced every 24 h for 72 h.
Cellproliferation assay
Cell proliferation was assessed by the MTSassay in accordance with the manufacturer's instructions. Briefly, pipet 20μlof Cell Titer 961AQueous One Solution Reagent containing a novel tetrazoliumcompound into each well of the 96-well assay plate containing the samples in100μl of culture medium, incubate the plate at 37oC for 2 hours in ahumidified, 5% CO2 atmosphere, the tetrazolium compound isbioreduced by metabolically active cells into a colored formazan product thatis soluble in tissue culture medium, record the absorbance at 490nm using a96-well plate reader. The normalized cell proliferation is calculated as thepercentage of measured absorbance of cell viability to that of control group(100%).
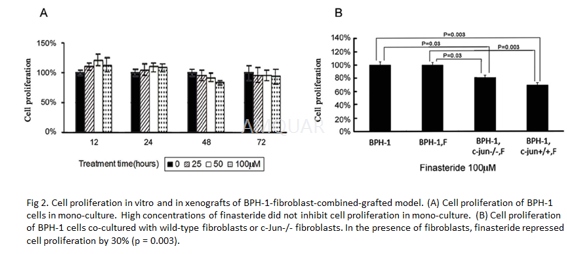
-
动物实验
Animals[6]
This study was conducted on male (n=180,275–300 g) and female (n=21, 275–300 g) Sprague Dawley rats. Animals were housed3–4 per cage in standard conditions.
Drugs
Finasteride (FIN) was suspended in a vehicle (VEH) solution containing 5%Tween80 and 95% sterile saline (SAL; 0.9% NaCl). Apomorphine hydrochloride was dissolvedin SAL containing 0.1% (v/v) ascorbic acid to prevent oxidation. SKF-82958hydrochloride was dissolved in distilled water. Ropinirole was dilutedin SAL.6-OHDAwas dissolved in SAL plus 0.02% ascorbic acid, and locally infused intothe medial forebrain bundle (MFB). L-DOPA methyl-ester and benserazide were dissolved in SAL. A 20:1 mixture of Fentanestand Domitor® in a volume range of 1.4–1.6 ml, IP, was used to induce generalanaesthesia. Antisedan® (0.37 mg/kg) was injected to reverse the sedativeeffect of the anesthetics.
Experimentaldesign
The first set of experiments was aimed atinvestigating the acute effects of FIN on LIDs in 6-OHDA-lesionedL-DOPA primed rats. After 3 weeks from the 6-OHDA injection, rats receiveddaily L-DOPA/ benserazide (6/6mg/kg, SC) treatment for 3 further weeks, so toinduce stable dyskinesias. Animals were then assigned to 3 treatment subgroups withequivalent average AIMs scores, and acutely injected with vehicle (VEH,Sal/Tween80) or different doses of FIN (30–60 mg/kg, IP), 40 min prior toL-DOPA treatment (6 mg/kg plus benserazide6 mg/kg, SC).
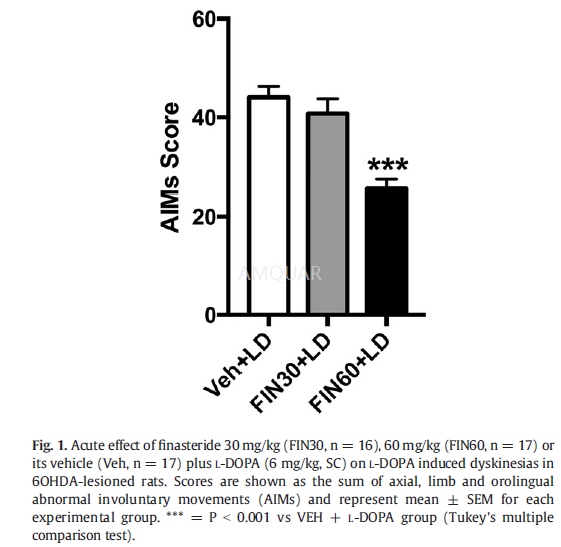
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Wasson KM WS. Proscar (Finasteride) inhibits 5 alpha-reductase activity in the ovaries and testes of Lytechinus variegatus Lamarck (Echinodermata: Echinoidea). Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;120(3):425-431.
[2] Clark RL AJ, Grossman SJ, Wise LD, Anderson C, Bagdon WJ, Prahalada S, MacDonald JS, Robertson RT. External genitalia abnormalities in male rats exposed in utero to finasteride, a 5 alpha-reductase inhibitor. Teratology. 1990;42(1):91-100.
[3] Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179-2184.
[more]
分子式
C23H36N2O2 |
分子量
372.54 |
CAS号
98319-26-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
75 mg/mL |
Water
0.05 mg/mL |
Ethanol
75 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01133444 | Healthy | Drug: Finasteride | Dr. Reddy's Laboratories Limited | Phase 1 | 2002-04-01 | 2010-07-09 |
| NCT01133457 | Healthy | Drug: Finasteride | Dr. Reddy's Laboratories Limited | Phase 1 | 2002-04-01 | 2010-05-27 |
| NCT01264289 | Healthy | Drug: Finasteride | Dr. Reddy's Laboratories Limited | Phase 1 | 2006-11-01 | 2010-12-20 |
| NCT01264302 | Healthy | Drug: Finasteride | Dr. Reddy's Laboratories Limited | Phase 1 | 2006-11-01 | 2010-12-20 |
| NCT00542243 | Enlarged Prostate | Drug: Finasteride|Drug: Placebo | University Health Network, Toronto|Merck Frosst Canada Ltd. | Phase 3 | 2008-02-01 | 2015-12-07 |
| NCT01296672 | Prostate Cancer | Drug: Finasteride|Drug: Placebo | The University of Texas Health Science Center at San Antonio|National Institutes of Health (NIH)|National Cancer Institute (NCI) | Phase 4 | 2011-02-01 | 2017-03-14 |
| NCT01585441 | Retinal Disease | Drug: Finasteride|Drug: Placebo | National Eye Institute (NEI)|The EMMES Corporation|National Institutes of Health Clinical Center (CC) | Phase 2 | 2012-04-01 | 2014-11-25 |
| NCT02548117 | ERYTHROCYTOSIS | Drug: Finasteride | Baylor College of Medicine | Phase 3 | 2016-02-01 | 2016-07-14 |
| NCT00837252 | Retinal Disease | Drug: Finasteride | National Eye Institute (NEI)|National Institutes of Health Clinical Center (CC) | Phase 1|Phase 2 | 2009-02-01 | 2016-09-22 |
| NCT01227993 | Retinal Disease | Drug: Finasteride | National Eye Institute (NEI)|National Institutes of Health Clinical Center (CC) | Phase 1|Phase 2 | 2010-10-01 | 2013-10-28 |
| NCT00600691 | Hematuria|Hematospermia | Drug: Finasteride | University of British Columbia|Health Canada|Merck Frosst Canada Ltd. | Phase 2 | 2008-03-01 | 2014-01-29 |
| NCT00564460 | Benign Prostatic Hyperplasia | Drug: Finasteride|Drug: Placebo | University of Alberta|Merck Frosst Canada Ltd. | Phase 3 | 2008-02-01 | 2009-11-04 |
| NCT00130767 | Benign Prostatic Hyperplasia | Drug: finasteride | Hospices Civils de Lyon | Phase 4 | 2004-12-01 | 2007-04-26 |
| NCT00564252 | Idiopathic Hirsutism | Drug: topical finasteride | Iran University of Medical Sciences|Firuzgar hospital affiliated to Iran University of Medical Sciences | | 2006-02-01 | 2007-11-26 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们