-
生物活性
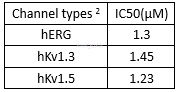
BAPTA-AM (BAPTA‑acetoxymethyl ester), a cell membranepermeable form of BAPTA that chelates Ca2+, is widely used as acell-permeant Ca2+chelator to study the role of Ca2+ in variousaspects of cell physiology. BAPTA-AM is a non-fluorescent, membrane-permeable form of BAPTA. Whereas the BAPTA, Free acid is not cell permeable and is only useful for manipulating extracellular Ca2+ levels, BAPTA-AM can be used with a wide variety of cells, where it is hydrolyzed by cytosolic esterases and trapped intracellularly. Blocks hKv1.5, hKV11.1 (hERG) and hKv1.3 channels (Ki values are 1.23, 1.30 and 1.45 μM respectively). Experiments have shown that BAPTA-AM abolishes vitamin D3-induced increase in intracellular Ca2+, and may induce inactivation of protein kinase C. Has also been shown to inhibit thapsigargin-induced apoptosis in rat thymocytes. This product is a high quality and sensitive compound for calcium signaling studies, investigation of signal transduction and apoptotic cascades, and neuroscience research.
TheIC50 value of BAPTA‑AM is determined as 13.6μMin the breast cancer 4T1 cell line.[1]
openchannel blocking effect of BAPTA‑AM[2]
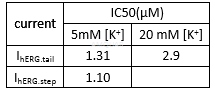
Effectsof BAPTA-AM on hERG channel current[2]
-
体外研究
-
体内研究
0.1% DMSO
-
激酶实验
-
细胞实验
Cell culture[1]
Mouse breast cancer 4T1 cell line wascultured in RPMI‑1640 (plus 4.5g/l glucose, 10mM HEPES, 1mMsodium pyruvate, 0.15% sodium bicarbonate, 100μg/ml streptomycin and 100U/ml penicillin)containing 10% FBS. Cell cultures were maintained at 37˚C in a 5% CO2 humidified incubator in 25cm2 Corning flasks. The cells weresubcultured at ~70% confluency
MTT‑based cytotoxicity assay
In total, 100,000 cells were seeded in35x10 mm Corning plates. In the logarithmic phase of growth, the cells weretreated with various doses of BAPTA‑AM and bortezomib(0.5μM BAPTA‑AM, 5μM BAPTA‑AM, 1nM bortezomib, 10nM bortezomib, 0.5μM BAPTA‑AM + 1nM bortezomib, 0.5μM BAPTA‑AM + 10nM bortezomib, 5μM BAPTA‑AM + 1nM bortezomib and 5μM BAPTA‑AM + 10nM bortezomib) for 24 h. Followinginhibitor exposure, the cells were treated for 24 h with RPMI‑1640 media containing 0.5% FBS + 0.5 mg/ml MTT at 37 ˚C with 5% CO2.Subsequently, the cells were incubated with 3% SDS (200μl) + 40mM HCl/isopropanol (1ml) for 15min inorder to dissolve the MTT‑formazan crystals.The absorbance of each sample was recorded at 570nm. Cell survival wasdetermined by analyzing the data with GraphPad Prism 3.03 software.
Halfmaximal inhibitory concentration (IC50) determination
MTT assay was performed as previouslydescribed to determine the IC50 of BAPTA‑AM on 4T1 cells. Thecells were treated with 100 and 500nM and 1, 10, 50 and 100μM BAPTA‑AM. The IC50 value of BAPTA‑AM was obtained byfitting the data with a GraphPad Prism 3.03 program to a sigmoidal dose‑response curve.

-
动物实验
Animals and housing[3]
Naive male Swiss mice and naive maleC57BL/6J were used in this study. Mice aged 4 weeks were housed three per cagefor locomotor activity experiments and acclimated to the colony room at least 2weeks prior to initiation of the study. For the Drinking in the Dark (DID)protocol, mice were housed four per cage for the first 2 weeks, and thentransferred to individual cages for 1 week prior to the beginning of theexperiment. Animals had free access to rodent chow and tap water. Roomtemperature was maintained at 22±1 °C and humidity (50%) in a 12/12-h light–darkcycle (lights on at 8:00 a.m.). For the DID experiments, a reverse light/darkcycle was used in which lights turned on at7:00 p.m. and off at 7:00 a.m.hours. The experiments started 3 h after the lights were shut off. Redincandescent lamps were kept on during the dark phase so that investigatorscould handle mice.
Drugs
Ethanol stocked at 96%, was diluted to 20%(v/v) in 0.9% (w/v) physiological saline. BAPTA-AM caffeine, cocainehydrochloride, Cremophor® EL, D-amphetamine sulfate and pentobarbital wereadministered in an injection volume of 10 ml/kg except for the ethanol-doseresponse experiments where the volume of the injection was adjusted to theethanol dose (0, 1.5, 2.5, 3.5 g/kg). All solutions were administeredintraperitoneally (i.p.) and prepared fresh daily in vehicle solution. BAPTA-AMwas diluted in Cremophor® EL 1.25% (v/v) in distilled water.
Locomotorstimulation induced by ethanol
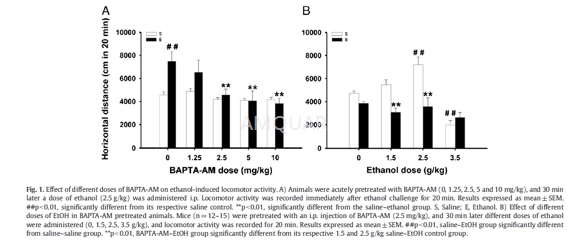
For locomotor activity experiments subjectswere moved from their home cages to the locomotor activity testing room atleast30–45 min before experiment initiation to allow acclimation toenvironmental conditions. Testing occurred between 10 a.m. and 4 p.m. Differentindependent experimental phases were conducted to assess the effects ofBAPTA-AM on drug-induced behavioral stimulation with a focus on the effects ofethanol. Experiment 1 assessed the effect of BAPTA-AM (0, 1.25, 2.5, 5 and 10mg/kg) on ethanol-induced locomotor activity. Mice (n=12–15) were injected witheither the vehicle or one of the four doses of BAPTA-AM and returned to theirhome cages for a waiting period of30 min. After this time, a challenge dose of0 or 2.5 g/kg of ethanol was administered immediately prior to placing animalsin the open field, where horizontal activity was measured for 20 min.Experiment2 studied the effects of several ethanol doses (0, 1.25, 2.5 and 3.5g/ kg) on locomotion in animals (n=12–15) pretreated with BAPTAAM (0 or 2.5mg/kg). This ethanol dose range was designed to evaluate the BAPTA-AM role fromthe stimulant-to-sedative effects of ethanol. A third experiment was conductedto explore the temporal pattern of the effect of BAPTA-AM on ethanol-inducedstimulation. Animals (n=12–15) were pretreated with BAPTA-AM (0 or 2.5 mg/kg)0, 30, 60 or 90 min before ethanol (2.5 g/kg). Experiment 4 evaluated whetherthe effects of BAPTA-AM found in previous experimental phases on ethanolstimulation are shared by other drugs with psychomotor properties. Mice(n=10–12) were injected with BAPTA-AM (0 or 2.5 mg/kg) and30 min before cocaine(4 mg/kg) amphetamine (2 mg/kg) or caffeine (15 mg/kg) administration. Animalswere placed immediately in the open field for 20 min to measure the locomotoractivity. Doses of amphetamine, caffeine and cocaine, were selected inaccordance with previous studies.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Yerlikaya A, Erdogan E, Okur E, Yerlikaya S, Savran B. A novel combination treatment for breast cancer cells involving BAPTA-AM and proteasome inhibitor bortezomib. Oncol Lett. 2016;12(1):323-330.
[2] Tang Q, Jin MW, Xiang JZ, et al. The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol. 2007;74(11):1596-1607.
[more]
分子式
C34H40N2O18 |
分子量
764.68 |
CAS号
126150-97-8 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
25 mg/mL |
Water
<1 mg/mL |
Ethanol
5 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们