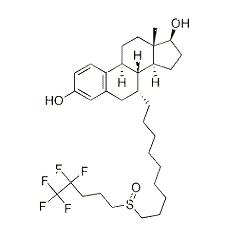
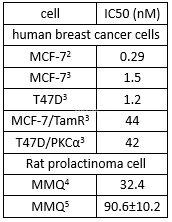
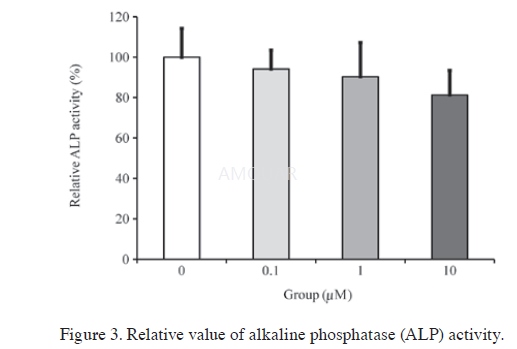
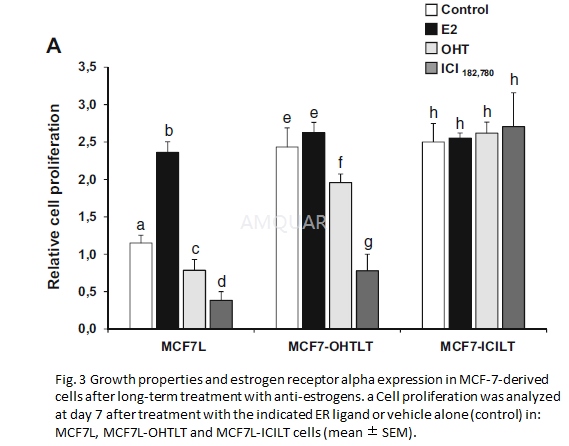
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00585507 | Breast Cancer | Drug: Fulvestrant | Beth Israel Deaconess Medical Center|Dana-Farber Cancer Institute|Brigham and Women's Hospital|Massachusetts General Hospital|Lowell General Hospital|University of Colorado, Denver|University of Maryland Greenebaum Cancer Center|South Shore Hospital | Phase 2 | 2004-04-01 | 2016-01-20 |
| NCT02953860 | Breast Cancer | Drug: Fulvestrant with Enzalutamide | University of Colorado, Denver|United States Department of Defense | Phase 2 | 2017-01-01 | 2017-01-26 |
| NCT01004419 | Carcinoma, Non Small Cell Lung | Drug: ZD6474 (vandetanib)|Drug: Faslodex (Fulvestrant) | University of Wisconsin, Madison|AstraZeneca|University of Pittsburgh | Phase 1 | 2009-11-01 | 2015-09-30 |
| NCT00617188 | Ovarian Cancer | Drug: Fulvestrant | Masonic Cancer Center, University of Minnesota | Phase 2 | 2007-06-01 | 2012-11-06 |
| NCT01597388 | Advanced Metastatic Breast Cancer | Drug: AZD2014|Drug: Fulvestrant | AstraZeneca | Phase 1 | 2012-05-01 | 2017-01-23 |
| NCT02795039 | Healthy | Drug: Fulvestrant|Drug: Fulvestrant | Fresenius Kabi | Phase 1 | 2016-06-01 | 2017-03-08 |
| NCT02955394 | Breast Cancer | Drug: Enzalutamide|Drug: Fulvestrant | University of Colorado, Denver|United States Department of Defense | Phase 2 | 2017-01-01 | 2017-01-26 |
| NCT01560416 | Breast Cancer | Drug: Fulvestrant|Drug: Ganetespib | Dana-Farber Cancer Institute | Phase 2 | 2012-05-01 | 2017-01-17 |
| NCT02540330 | Female Breast Carcinoma|Female Ductal Carcinoma In Situ | Drug: Fulvestrant | Atossa Genetics, Inc. | Phase 2 | 2016-03-01 | 2016-10-18 |
| NCT00476645 | Prostatic Neoplasms|Prostate Cancer | Drug: Fulvestrant | Stanford University|AstraZeneca | Phase 2 | 2006-09-01 | 2014-08-04 |
| NCT02909361 | Metastatic Breast Cancer | Drug: Fulvestrant | Fudan University | | 2017-03-01 | 2017-02-22 |
| NCT01300351 | Breast Cancer | Drug: Fulvestrant|Drug: Placebo | AstraZeneca | Phase 3 | 2011-03-01 | 2015-04-23 |
| NCT02738866 | Metastatic Breast Cancer | Drug: Palbociclib|Drug: Fulvestrant | Sidney Kimmel Comprehensive Cancer Center|Pfizer | Phase 2 | 2016-08-01 | 2017-02-06 |
| NCT02383030 | Metastatic Breast Cancer | Drug: Fulvestrant | Consorzio Oncotech|Clinical Research Technology S.r.l. | Phase 3 | 2015-11-01 | 2016-06-14 |
| NCT02374099 | Breast Neoplasms | Drug: CC-486|Drug: Fulvestrant | Celgene Corporation | Phase 2 | 2015-03-01 | 2016-10-25 |
| NCT00328120 | Advanced Breast Cancer | Drug: Fulvestrant | AstraZeneca | Phase 1 | 2004-04-01 | 2010-11-16 |
| NCT00921115 | Invasive Breast Cancer | Drug: Fulvestrant|Drug: Anastrazole | University of Kansas Medical Center|AstraZeneca | Phase 2 | 2009-05-01 | 2017-01-20 |
| NCT01160718 | Breast Cancer | Drug: fulvestrant|Drug: selumetinib | Swiss Group for Clinical Cancer Research | Phase 2 | 2010-08-01 | 2016-09-16 |
| NCT00201864 | Breast Cancer | Drug: Exemestane|Drug: Fulvestrant | Ewa Mrozek|Pfizer|Ohio State University Comprehensive Cancer Center | Phase 2 | 2005-09-01 | 2016-07-14 |
| NCT00570921 | Breast Cancer | Drug: Everolimus|Drug: Fulvestrant | Mara Chambers|Novartis|University of Kentucky | Phase 2 | 2008-04-01 | 2017-01-08 |
| NCT00660803 | Breast Cancer | Drug: Fulvestrant | AstraZeneca|Dendrix - Scientific Information Architecture | | 2008-05-01 | 2010-01-20 |