-
生物活性
TSU-68 (SU6668, Orantinib), a potent,orally administered, small-molecule inhibitor of the angiogenic relatedreceptor tyrosine kinases Flk-1/KDR, PDGFRβ, and FGFR1, hasbeen developed as an angiogenesis inhibitor in oncotherapy, especially forsolid tumor treatment. TSU-68 is an ATP-competitive PDGFR, VEGF and FGFR inhibitor (IC50 values are 0.06, 2.43, 3.04 and > 100 μM at PDGFRβ, VEGFR2, FGFR1 and EGFR respectively). TSU-68 inhibits proliferation of HUVEC and NIH3T3 cells in vitro (IC50 values are 0.41, 9.3 and 16.5 μM for Flk-1/KDR, FGF and PDGF-stimulated growth respectively) and induces > 75% growth inhibition against a broad range of tumor types. TSU-68 shows antiangiogenic, anti-inflammatory, antimetastatic and proapototic activity. Exhibits antiangiogenic, anti-inflammatory, antimetastatic and proapoptotic activity.
Kinase activities of SU6668

phosphorylation of SU6668

Anti-proliferation of SU6668
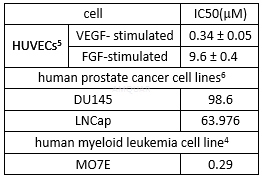
Cytotoxicityof SU6668[7]

-
体外研究
-
体内研究
1% DMSO+30% polyethylene glycol+1% Tween 80
-
激酶实验
Biochemical Tyrosine Kinase Assays[5]
RecombinantProtein Production
GST-fusion proteins of FGFR1 (kinase domain)and Flk-1 (cytoplasmic domain) were produced in the baculovirus expressionsystem. For both constructs, pFBG2T was used as the transfer vector. Thisplasmid contains the GST coding sequence, which was amplified by PCR as aBamHI/BglII fragment and cloned into the BamHI site of pFastBac-1. The portionof the FGFR1 cDNA encoding amino acids 459–757 was amplified by PCR as an EcoRI/HindIIIfragment and ligated downstream of and in frame with the GST coding sequence inpFBG2T. The portion of Flk-1 cDNA encoding amino acids 812-1346 was amplifiedby PCR as a NotI/SphI fragment and ligated downstream of and in frame with theGST coding sequence in pFBG2T. Recombinant viruses containing the differentrecombinant transfer vectors were produced following standard protocols. Forprotein production, Sf9 cells were infected following standard procedures, andfusion proteins were purified by affinity chromatography onglutathione-Sepharose. GST-fusion preparations were determined to be of highquality, with no detectable breakdown products.
trans-PhosphorylationReactions
Biochemical tyrosine kinase assays to quantitatethe trans-phosphorylation activity of Flk-1 and FGFR1 were performed in 96-wellmicrotiter plates precoated (20μg/well in PBS; incubated overnight at 4°C) with the peptidesubstrate poly-Glu, Tyr (4:1). Excess protein binding sites were blocked withthe addition of 1–5% (w/v) BSA in PBS. Purified GST-FGFR1 (kinase domain) orGST-Flk-1 (cytoplasmic domain) fusion proteins were produced inbaculovirus-infected insect cells. GSTFGFR1 and GST-Flk-1 were then added tothe microtiter wells in 2x concentration kinase dilution buffer consisting of100mM HEPES, 50mM NaCl, 40μM NaVO4, and 0.02% (w/v) BSA. The final enzyme concentration for GST-Flk-1and GST-FGFR1 was 50ng/ml. SU6668 was dissolved in DMSO at 100x the finalrequired concentration and diluted 1:25 in H2O. 25μl of dilutedSU6668 were subsequently added to each reaction well to produce a range of inhibitorconcentrations appropriate for each enzyme. The kinase reaction was initiatedby the addition of different concentrations of ATP in a solution of MnCl2 sothat the final ATP concentrations spanned the Km for the enzyme, and the finalconcentration of MnCl2 was 10mM. The plates were incubated for 5–15 min at roomtemperature before stopping the reaction with the addition of EDTA. The plateswere then washed three times with TBST. Rabbit polyclonal antiphosphotyrosineantisera were added to the wells at a 1:10,000 dilution in TBST containing 0.5%(w/v) BSA, 0.025% (w/v) nonfat dry milk, and 100μM NaVO4 andincubated for 1 h at 37°C. The plates were then washed three times with TBST,followed by the addition of goat anti rabbit antisera conjugated withhorseradish peroxidase (1:10,000 dilutions in TBST). The plates were incubatedfor 1 h at 37°C and then washed three times with TBST. The amount ofphosphotyrosine in each well was quantitated after the addition of2,2’-azino-di-[3-ethylbenzthiazoline sulfonate] as substrate.
AutophosphorylationReactions
Tyrosine kinase assays to quantitate the autophosphorylationactivity of PDGFR or EGFR were performed in a similar manner, except that thewells were precoated (0.5μg/well in 100μl of PBS) with PDGFRβ- or EGFR-specific monoclonal antibodies (28D4C10 and SUMO1,respectively) to capture the respective kinase from lysates of NIH-3T3 cellsengineered to overexpress PDGFRβ or EGFR. The reaction buffer for the autophosphorylation studiesconsisted of 25mM Tris, 100mM NaCl, 10mM MnCl2, 0.1% (v/v) Triton X-100, and0.5mM DTT.
The linear phase of each assay wasdetermined, and reaction rates were calculated from the linear phase of aseries of reactions whose duration spanned the linear period. Assays werehighly linear with respect to substrate concentration and time. Data wereanalyzed using the Lineweaver-Burk inverse-reciprocal plot of 1/rate versus1/ATP concentration. Ki calculations were made using the assumption that in thecase of competitive inhibition, Km is increased by a factor of (1 + [I]/Ki),where [I] is the concentration of inhibitor, and in the case of noncompetitiveinhibition, Vmax is decreased by a factor of (1 + [I]/Ki).
-
细胞实验
Cell cultures[6]
The human prostate cancer cell lines DU145,LNCap and PC3 were maintained in RPMI-1640 supplemented with 10% fetal bovineserum (FBS) and 1% penicillin/streptomycin. All cell lines used in the presentstudy were cultured in a humidified environment containing 5% CO2 and held at aconstant temperature of 37˚C.
Real-timePCR
Total RNA was extracted using Tri zol reagentand reverse transcribed using the transcriptase cDNA synthesis kit according tothe manufacturer's instructions. Real-time PCR analysis was performed usingSYBR Premix Ex Taq™ in an Applied Biosystems7500 Real-Time PCR system accordingto the manufacturer's instructions. A primer (F, AAGCAGTGCAAAACAGTTCACG and R,GCACCTTATCACGTTTACGCT) for MTDH mRNA expression detecting was used.
CellCounting kit-8
Cells were seeded in 96-well plates and theproliferation of the cells was assayed at 0, 24, 36 and 48 h using Cell Countingkit-8 (CCK-8) according to the manufacturer's instructions. Cell viability wasassessed by the measurement of absorbance at 450 nm using a microplate reader.
Westernblot analysis
Cells were treated in 6-well plates, washedthree times by phosphate-buffered saline (PBS) and lysed for 10 min on ice inradioimmunoprecipitation assay (RIPA) buffer containing an anti-proteasemixture. Protein concentration was measured by bicinchoninic acid assay (BCA).The protein fractions were resuspended in loading buffer and denatured at 100˚Cfor 10 min. Total proteins (20μg/lane) were separated on 10% SDS polyacrylamidegels and transferred to polyvinylidene fluoride (PVDF) membranes. The membraneswere then blocked in 5% fat-free milk Trisbuffered saline, 0.1% Tween-20 (TBST)buffer for 2 h at room temperature. Primary antibodies were used according tothe manufacturer's instructions.

-
动物实验
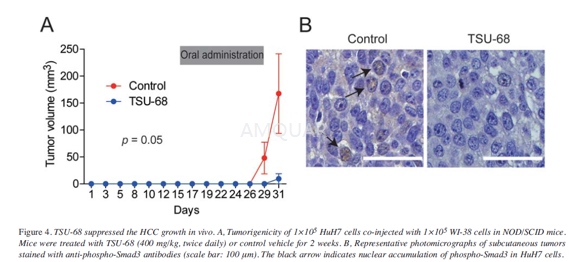
Tumorigenicity in non-obese diabetic/severecombined immuno -deficient (NOD/SCID) mice[8]
Cells (1×105 HuH7 and 1×105 WI-38 cells) were mixed and suspended in 200μl of 1:1 DMEM and Matrigel andsubcutaneously injected into 6- week-old NOD/SCID mice (NOD/NCrCRl-Prkdcscid).
Control vehicle (aqueous carboxymethylcellulosecontaining 0.9% (w/v) benzyl alcohol, 0.4% (w/v) Tween 80, and 9mg/ml sodiumchloride) or TSU-68 solution(400mg/kg) was orally ingested twice daily on days16-20 and 23-28. The sizes and incidence of subcutaneous tumors were recorded.All mice were euthanized on day 31 and tumors were formalin-fixed and paraffin-embeddedand used for immunohistochemistry.
Immunohistochemistryanalysis
Immunohistochemistry (IHC) was performedusing mouse anti-Smad3Lrabbit antibodies and Envision+ kits.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Krystal GW HS, Kiewlich D, Liang C, Vasile S, Sun L, McMahon G, Lipson KE. Indolinone tyrosine kinase inhibitors block Kit activation and growth of small cell lung cancer cells. Cancer Res. 2001;61(9):3660-3668.
[2] Godl K GO, Eickhoff J, Wissing J, Blencke S, Weber M, Degen H, Brehmer D, Orfi L, Horváth Z, Kéri G, Müller S, Cotten M, Ullrich A, Daub H. Proteomic characterization of the angiogenesis inhibitor SU6668 reveals multiple impacts on cellular kinase signaling. Cancer Res. 2005;65(15):6919-6926.
[3] Piirsoo A, Kasak L, Kauts ML, et al. Protein kinase inhibitor SU6668 attenuates positive regulation of Gli proteins in cancer and multipotent progenitor cells. Biochim Biophys Acta. 2014;1843(4):703-714.
[4] Smolich BD YH, West KA, Giles FJ, Albitar M, Cherrington JM. The antiangiogenic protein kinase inhibitors SU5416 and SU6668 inhibit the SCF receptor (c-kit) in a human myeloid leukemia cell line and in acute myeloid leukemia blasts. Blood. 2001;97(5):1413-1421.
[more]
分子式
C18H18N2O3 |
分子量
310.35 |
CAS号
252916-29-3 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
62 mg/mL |
Water
<1 mg/mL |
Ethanol
<1.2 mg/mL |
体内溶解度
约32 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00784290 | Hepatocellular Carcinoma | Drug: Orantinib (TSU-68) | Taiho Pharmaceutical Co., Ltd. | Phase 1|Phase 2 | 2003-09-01 | 2012-03-22 |
| NCT01465464 | Hepatocellular Carcinoma | Drug: Orantinib (TSU-68)|Drug: Placebo | Taiho Pharmaceutical Co., Ltd. | Phase 3 | 2010-12-01 | 2017-02-03 |
| NCT00024206 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: orantinib|Other: laboratory biomarker analysis|Other: pharmacological study | National Cancer Institute (NCI) | Phase 1 | 2001-07-01 | 2013-01-22 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们























