-
生物活性
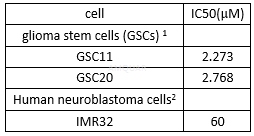
Tideglusib(NP-031112), a selective irreversible andnon-ATP-competitive GSK-3 inhibitor with good blood-brain barrier penetration, hasneuroprotective effects against neurodegenerative diseases in clinical trials. Tideglusib is an irreversible, non ATP-competitive GSK-3β inhibitor (IC50= 60 nM). Tideglusib irreversibly inhibits GSK-3, reduces tau phosphorylation, and prevents apoptotic death in human neuroblastoma cells and murineprimary neurons. Tideglusib inhibits glutamate-induced glial activation as evidenced by decreased TNF-α and COX-2 expression in rat primary astrocyte or microglial cultures.
Cell viability of NP-031112
GSK-3β inhibitionwith or without a preincubation of the enzyme
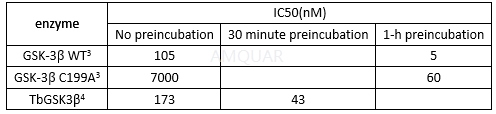
Tideglusibinhibits trypanosome proliferation with GI50 of 2.3μM[4]
-
体外研究
-
体内研究
-
激酶实验
Expression and purification of recombinantTbGSK3β[4]
Full-length TbGSK3β (Gene DB IDTb927.10.13780) was cloned into the pET21d expression vector in-frame with aC-terminal 6X His tag. BL21 (DE3) E. coli were transformed with the constructand cultured in terrific broth (TB) supplemented with 1% glucose to an OD (at590 nm) of 1.8. Expression of TbGSK3β was induced with IPTG (0.5mM) for 3 hoursat 32°C. Cells were harvested, and resuspended in lysis buffer (30mM HEPES(pH8.0), 300mM NaCl, 10% glycerol, 10mM imidazole, 0.2μM PMSF, and2mMβ-mercaptoethanol). The bacteria were lysed by sonication and freeze/thawcycles. Insoluble protein were removed by centrifugation (12000 x g, 45 min,4°C), and the soluble fraction loaded onto immobilized metal affinitychromatography (IMAC) column. The column was washed with 30mM HEPES (pH 8.0),0.5M NaCl, 10% glycerol, 20mM imidazole, 0.02% Triton X-100, 2mM β-mercaptoethanol.Recombinant TbGSK3β (TbGSK3β) was eluted from the IMAC column with 6mL of 30mMHEPES (pH 7.5), 300mM NaCl, 10% glycerol, 200mM imidazole, 2mM β-mercaptoethanol.Eluted TbGSK3β was further fractionated by size-exclusion chromatography (SEC)using a Superdex-200, 10/300 GL column pre-equilibrated with SEC runningbuffer, 50mM Tris-HCl (pH 7.5), 100mM NaCl. Fractions were collected, a portionof each was analyzed by SDS-PAGE to determine which fraction contained TbGSK3β.A band corresponding to the molecular weight of rTbGSK3β eluted in fractions 13–15,and was considered the purified enzyme. From a 12 x 1 liter of culture 136 mgof protein was recovered at 3.8 mg/ml. Purified TbGSK3β was stored at -80°C in50mM Tris-HCl (pH 7.5), 100mM NaCl, 20% glycerol until used.
Enzymeassay
TbGSK3β activity was measured by followingthe ADP formation associated with the phosphorylation of GSM peptide anddetected with ADP-Glo at 23°C. The enzyme reaction was conducted in a volume of20μL in a buffer containing 20mM NaCl, 15mM HEPES pH 7.4, 1mM EGTA, 10mM MgCl2,0.02% Tween. These assays contained GSMat10μM (approximating Km) or 80μM(approximating Vmax). ATP was added at 10μM and100μM to approximate Km andVmax, respectively.
All reactions were incubated for 5 minutesin microcentrifuge tubes, and reactions were terminated at 80°C for 4 min. Of20μL reactions, 17.5μL were transferred into 384 well-plates. Promega ADP-Gloprocedures were carried out and phosphorylation measured by Luminoskan Ascent.For pre-incubation experiments, TbGSK3β was preincubated at room temperaturewith inhibitor, GSM and buffer for 30 min. Inhibitors were added in DMSO to afinal concentration of DMSO in the reactions of <5%. The kinase reaction wasthen started by adding ATP. For non-preincubation reactions, the kinasereactions were started by adding TbGSK3β to a mixture containing inhibitor,ATP, GSM and buffer. DMSO was added to all control reactions. All compoundsevaluated in this study were tested for their ability to inhibit the ADP-Glodetection assay in experiments with ADP present and no TbGSK3β and did notinhibit this reaction up to 100μM.

-
细胞实验
Cellculture
IMR32 (human neuroblastoma cell line) were maintainedat 37◦C with 5% CO2,supplemented with DMEM, 10%FBS and 1%Antibiotic/Antimycotic solution according to manufac-turer’s instructions.IMR32 cells were maintained from passage 7and discarded with a maximum of 23passages.
Cellviability colorimetric assay (MTT)
The cell viability of tideglusib (TDG)-treatedcells were determined by 3-(4, 5-dimethylthiazol- 2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay using the EZcountTM MTT Cell AssayKit. Healthy viable IMR32 cells at 104cells/well were seeded in a96-well microtitre culture plate under typical culture conditions. Cells wereallowed to attach overnight and later washed with 1X PBS followed by individualtreatments with various doses of TDG (5μM–120μM)for48 h. The assay was performed according to the manufacturer’s protocol andthe absorbance was read at 650 nm in an ELISA iMARKTM microplatereader. The effect of TDG on the viability of IMR32 cells was performed bymeasuring% viability = A570 of treated cells/A570 of control cells × 100. Theinhibitory concentration (IC50) value of both drugs was determined by plottinga dose-response graph.
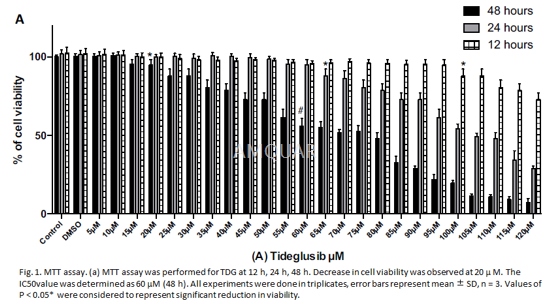
-
动物实验
Intracranial tumor cell injection[1]
GSC11 or GSC20 cells (5×105/ mouse)were injected intracranially into 6- to 8-week-old male nude (nu/nu) mice(5mice for each group). At the end of the experiments, the mice were humanelykilled, and each mouse’s brain was harvested, fixed in 4% formaldehyde, andembedded in paraffin. Tumor formation was determined by histologic analysis oftissue sections stained with hematoxylin and eosin. Tumor volumes werecalculated using the formula V= (π/6) ×a2×b, where a and b are thetumor’s short axis and long axis, respectively.
For in vivo therapeutic experiments, 1 dafter intracranial implantation of tumor cells into nude mice (8 mice for eachgroup), tideglusib (25mg/kg/d) or TMZ (20mg/kg/d) in a vehicle of dimethylsulfoxide/polyethylene glycol 300 (1:4 ratio) was injected intraperitoneallyevery other day for 30 d. For combinatorial treatment, mice received injectionsof TMZ or tideglusib on alternating days for 30 d. The vehicle alone was usedfor the negative control group. In a set experiment to analyze mouse survival,animals were humanely killed when they were moribund; the remaining animalswere humanely killed 90 d after tumor-cell injection.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Zhou A, Lin K, Zhang S, et al. Nuclear GSK3beta promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol. 2016;18(9):954-966.
[2] Mathuram TL, Ravikumar V, Reece LM, Karthik S, Sasikumar CS, Cherian KM. Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G0/G1 accumulation and ROS generation. Environ Toxicol Pharmacol. 2016;46:194-205.
[more]
分子式
C19H14N2O2S |
分子量
334.39 |
CAS号
865854-05-3 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
54 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约3 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02586935 | Autism Spectrum Disorders | Drug: Tideglusib|Other: Placebo | Evdokia Anagnostou|Holland Bloorview Kids Rehabilitation Hospital|McMaster University|University of Western Ontario, Canada|St. Michael's Hospital, Toronto|University of Toronto|Anagnostou, Evdokia, M.D. | Phase 2 | 2015-12-01 | 2017-03-17 |
| NCT01350362 | Alzheimer's Disease | Drug: tideglusib|Drug: tideglusib|Drug: tideglusib|Drug: Placebo | Noscira SA|ICON Clinical Research | Phase 2 | 2011-04-01 | 2012-10-01 |
| NCT02858908 | Myotonic Dystrophy 1 | Drug: Tideglusib | AMO Pharma Limited | Phase 2 | null | 2017-03-15 |
| NCT01049399 | Progressive Supranuclear Palsy | Drug: tideglusib|Drug: tideglusib|Drug: placebo | Noscira SA|i3 Research | | 2009-12-01 | 2012-01-02 |
| NCT00948259 | Alzheimer麓s Disease | Drug: NP031112|Drug: Placebo | Noscira SA | Phase 1|Phase 2 | 2008-12-01 | 2009-11-10 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们






















