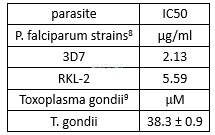
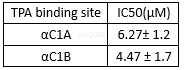
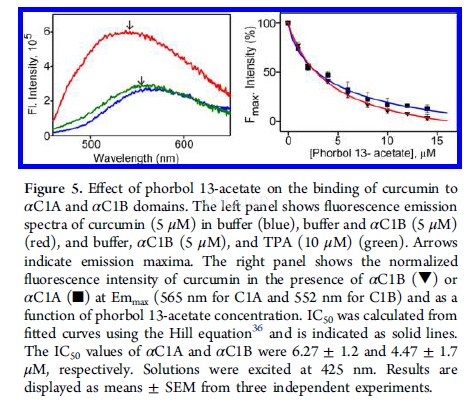
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01052025 | Type 2 Diabetes|Pre-diabetes|Insulin Resistance|Cardiovascular Risk | Drug: Curcumin | Srinakharinwirot University|Ministry of Health, Thailand | Phase 4 | 2009-08-01 | 2010-01-19 |
| NCT01179256 | Atopic Asthma | Dietary Supplement: CURCUMIN|Other: no intervention other than stopping study | University of South Florida | | 2009-03-01 | 2010-08-10 |
| NCT01383161 | Age-associated Cognitive Impairment|Mild Cognitive Impairment (MCI) | Drug: Curcumin|Other: Placebo | University of California, Los Angeles | Phase 2 | 2012-03-01 | 2015-12-02 |
| NCT03085680 | Older Adults|Physical Function|Cognitive Function | Drug: Curcumin|Drug: microcrystalline cellulose | University of Florida|National Institute on Aging (NIA) | Phase 2|Phase 3 | 2017-04-01 | 2017-03-23 |
| NCT01246973 | Radiation-induced Dermatitis | Drug: Curcumin|Drug: Placebo | University of Rochester|National Cancer Institute (NCI) | Phase 2|Phase 3 | 2011-02-01 | 2016-02-08 |
| NCT02944578 | Neoplasms | Drug: Curcumin|Drug: Placebo | Emory University | Phase 2 | 2016-10-01 | 2016-10-24 |
| NCT01333917 | Colorectal Cancer | Drug: Curcumin C3 tablet | University of North Carolina, Chapel Hill | Phase 1 | 2010-11-01 | 2013-02-06 |
| NCT02645656 | Oral Submucous Fibrosis | Drug: Curcumin Arm | SVS Institute of Dental Sciences | Phase 2 | 2013-12-01 | 2016-01-04 |
| NCT01035580 | Uterine Cervical Dysplasia | Drug: Curcumin|Drug: curcumin | Emory University | Phase 1 | 2010-01-01 | 2013-11-27 |
| NCT02321293 | Lung Cancer | Dietary Supplement: CurcuVIVA鈩Drug: Tyrosine Kinase Inhibitor gefitinib (Iressa)|Drug: Tyrosine Kinase Inhibitor erlotinib (Tarceva) | Lady Davis Institute|Jewish General Hospital | Phase 1 | 2015-08-01 | 2015-09-01 |
| NCT01294072 | Colon Cancer | Dietary Supplement: curcumin|Dietary Supplement: Curcumin conjugated with plant exosomes|Other: No intervention | James Graham Brown Cancer Center|University of Louisville | Phase 1 | 2011-01-01 | 2017-03-01 |
| NCT00895167 | Healthy | Dietary Supplement: curcumin | Daniel Doberer|Medical University of Vienna | Phase 1 | 2009-01-01 | 2013-01-16 |
| NCT01052597 | Type 2 Diabetes Mellitus|Cardiovascular Abnormalities | Drug: curcumin | Srinakharinwirot University|Ministry of Health, Thailand | Phase 4 | 2009-07-01 | 2010-01-19 |
| NCT00927485 | Familial Adenomatous Polyposis | Drug: Calcumin (Curcumin)|Other: Risk Factor Questionnaire|Other: Blood samples|Other: Biopsies (Sigmoidoscopy)|Other: Biopsies (Upper endoscopy) | University of Puerto Rico | | 2007-11-01 | 2016-12-12 |
| NCT02255370 | Crohn's Disease | Drug: Curcumin | University Hospital, Clermont-Ferrand|3i nature|Naturop么le Nutrition sant茅 | Phase 3 | 2014-12-01 | 2016-07-25 |
| NCT02476708 | Schizophrenia|Schizoaffective Disorder | Dietary Supplement: curcumin 1800mg|Dietary Supplement: Placebo | Yale University | Phase 2 | 2016-02-01 | 2016-06-13 |
| NCT02104752 | Schizophrenia|Cognition|Psychosis | Drug: Curcumin | VA Greater Los Angeles Healthcare System|Stanley Medical Research Institute|Theravalues, Inc.|University of California, Los Angeles | Phase 1|Phase 2 | 2014-07-01 | 2014-07-19 |
| NCT03016039 | Curcumin Use for Gynecological Conditions | Dietary Supplement: Curcumin supplementation | Meir Medical Center | | 2017-01-01 | 2017-01-11 |
| NCT02298985 | Chronic Schizophrenia | Drug: Curcumin|Drug: placebo | Beersheva Mental Health Center|Tirat Carmel Mental Health Center | Phase 4 | 2015-01-01 | 2016-01-12 |
| NCT01811381 | Mild Cognitive Impairment | Drug: Curcumin|Behavioral: aerobic yoga|Behavioral: non aerobic yoga|Dietary Supplement: Placebo | VA Office of Research and Development | Phase 2 | 2014-01-01 | 2017-01-17 |
| NCT01403545 | Drug Safety | Drug: Liposomal Curcumin|Other: Placebo | SignPath Pharma, Inc. | Phase 1 | 2011-08-01 | 2014-05-23 |
| NCT01875822 | Schizophrenia|Schizoaffective Disorder|Depression | Dietary Supplement: Super-Curcumin | Woodbury, Michel, M.D.|Lawson Health Research Institute | Phase 1|Phase 2 | 2009-06-01 | 2013-06-09 |
| NCT00595582 | Mild Cognitive Impairment | Dietary Supplement: curcumin + bioperine | Louisiana State University Health Sciences Center Shreveport | | 2005-05-01 | 2012-05-25 |
| NCT02064673 | Prostate Cancer | Drug: Curcumin|Drug: placebo | University of Texas Southwestern Medical Center | Phase 2 | 2014-05-01 | 2016-05-31 |