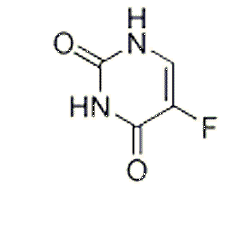
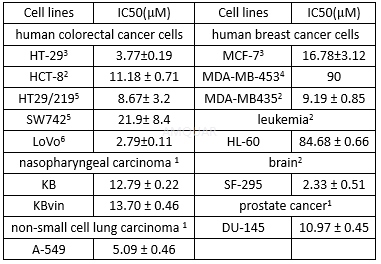
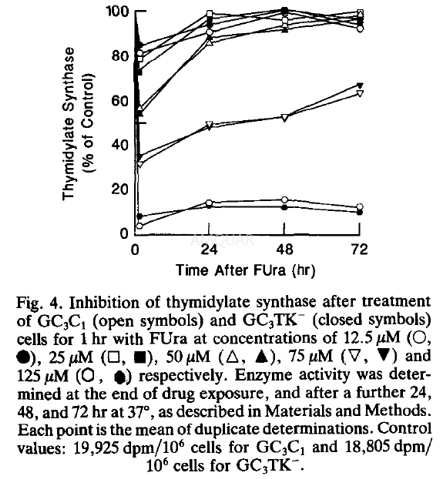
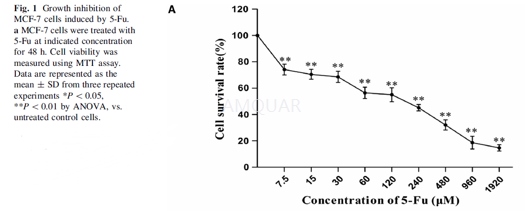
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01498783 | Central Nervous System Malignancies|Ependymoma | Drug: 5-fluorouracil | St. Jude Children's Research Hospital | Phase 1 | 2011-12-01 | 2016-02-01 |
| NCT02557503 | Advanced Adult Primary Liver Cancer | Drug: Oxaliplatin and fluorouracil | Zhu Xu|Beijing Cancer Hospital | Phase 4 | 2015-01-01 | 2015-09-22 |
| NCT01953406 | Hepatocellular Carcinoma|Lung Metastasis | Drug: 5-fluorouracil|Drug: Mitomycin | Seoul National University Hospital | Phase 2 | 2015-11-01 | 2016-04-26 |
| NCT00090025 | Biliary Tract Cancer | Drug: becatecarin|Drug: 5-Fluorouracil Plus Leucovorin | Helsinn Healthcare SA | Phase 3 | 2004-09-01 | 2009-01-13 |
| NCT01740648 | Recurrent Rectal Cancer|Stage IIA Rectal Cancer|Stage IIB Rectal Cancer|Stage IIC Rectal Cancer|Stage IIIA Rectal Cancer|Stage IIIB Rectal Cancer|Stage IIIC Rectal Cancer | Drug: trametinib|Drug: fluorouracil|Radiation: radiation therapy | Evan Wuthrick|National Comprehensive Cancer Network|Novartis Pharmaceuticals|Ohio State University Comprehensive Cancer Center | Phase 1 | 2012-11-01 | 2016-11-14 |
| NCT00033735 | Pancreatic Cancer | Drug: fluorouracil|Drug: Irofulven | Eisai Inc. | Phase 3 | 2000-01-01 | 2012-12-20 |
| NCT01954992 | Metastatic Pancreatic Adenocarcinoma | Drug: Glufosfamide|Drug: Fluorouracil | Eleison Pharmaceuticals LLC. | Phase 3 | 2013-09-01 | 2017-02-23 |
| NCT01658813 | Gastrointestinal Cancer Metastatic|Renal Cell Cancer Metastatic|Non Small Cell Lung Cancer Metastatic | Drug: 5-Fluorouracil and Interferon | Western Regional Medical Center | Phase 2 | 2012-07-01 | 2016-11-10 |
| NCT01055743 | Early Stage Hepatocellular Carcinoma | Procedure: Radical resection|Drug: Fluorouracil Implants | Simcere Pharmaceutical Co., Ltd|Eastern Hepatobiliary Surgery Hospital | Phase 2 | 2009-08-01 | 2010-01-24 |
| NCT02705352 | Ectropion|Skin Neoplasms | Drug: 5-Fluorouracil|Other: Normal saline | Massachusetts Eye and Ear Infirmary | Early Phase 1 | 2016-10-01 | 2016-09-12 |
| NCT00696488 | Actinic Keratosis | Drug: Fluorouracil 0.5% | Wake Forest University|Wake Forest University Health Sciences | Phase 4 | 2007-04-01 | 2009-02-12 |
| NCT00110721 | Colorectal Cancer | Drug: GM-CT-01 plus 5-Fluorouracil | Galectin Therapeutics Inc. | Phase 2 | 2005-05-01 | 2012-03-05 |
| NCT00390416 | Stomach Neoplasms|Esophageal Neoplasms | Drug: Docetaxel, Cisplatin, Fluorouracil, Bevacizumab, Leucovorin | Memorial Sloan Kettering Cancer Center|Sanofi|Genentech, Inc. | Phase 2 | 2006-10-01 | 2016-02-16 |
| NCT00943137 | Cancer (Advanced Stage) | Drug: 5-Fluorouracil | National University Hospital, Singapore | Phase 2 | 2009-06-01 | 2015-06-17 |
| NCT00557557 | Colorectal Liver Metastases | Drug: Drug: 5-FU|Drug: Drug: Oxaliplatin | David Bartlett|National Institutes of Health (NIH)|National Cancer Institute (NCI)|University of Pittsburgh | Phase 1 | 2007-07-01 | 2015-12-23 |
| NCT02523053 | Hepatocellular Carcinoma|Recurrence | Procedure: Hepatectomy|Drug: 5-Fluorouracil | Eastern Hepatobiliary Surgery Hospital | | 2016-04-01 | 2016-10-20 |
| NCT01317069 | Gallbladder Cancer|Bile Duct Cancer | Drug: Fluorouracil implant | Simcere Pharmaceutical Co., Ltd|Eastern Hepatobiliary Surgery Hospital | Phase 2 | 2010-06-01 | 2011-03-15 |
| NCT00611754 | Head and Neck Cancer | Drug: Oxaliplatin, 5-FU | Sanofi | Phase 2 | 2000-05-01 | 2008-01-28 |
| NCT01661114 | Pancreatic Cancer|Biliary Cancer | Drug: Gemcitabine|Drug: 5-FU|Drug: Cisplatin | University of Michigan Cancer Center | Phase 2 | 2011-07-01 | 2016-09-01 |
| NCT00388700 | Colorectal Cancer | Drug: GM-CT-01|Drug: 5-Fluorouracil, Leukovorin, bevacizumab | Galectin Therapeutics Inc. | Phase 2 | 2006-10-01 | 2016-08-08 |