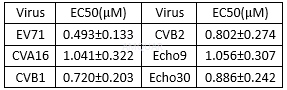
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02652871 | Leukemia | Drug: LY2510924|Drug: Idarubicin|Drug: Cytarabine | M.D. Anderson Cancer Center|Eli Lilly and Company|High Impact Clinical Research Support Program | Phase 1 | 2016-05-01 | 2017-01-05 |
| NCT01518556 | Leukemia, Myeloid, Acute | Drug: Idarubicin | Konkuk University Medical Center|Chung-Ang University Hosptial, Chung-Ang University College of Medicine|Seoul National University Hospital|Ewha Womans University|Samsung Medical Center|Chonbuk National University Hospital|Pusan National University Hospital|Hanyang University Seoul Hospital|Soon Chun Hyang University | Phase 1|Phase 2 | 2011-07-01 | 2016-09-01 |
| NCT01305135 | High Grade Myelodysplastic Syndrome Lesions | Drug: azacitidine and idarubicin | Groupe Francophone des Myelodysplasies | Phase 1|Phase 2 | 2010-12-01 | 2015-10-28 |
| NCT00074737 | Acute Myelogenous Leukemia | Drug: cenersen|Drug: Idarubicin|Drug: Cytarabine | Eleos, Inc. | Phase 2 | 2004-04-01 | 2014-09-16 |
| NCT00422591 | Leukemia|Acute Myeloid Leukemia|AML | Drug: Cytarabine|Drug: Idarubicin | M.D. Anderson Cancer Center | Phase 2 | 2006-12-01 | 2017-01-17 |
| NCT00528398 | Leukemia | Drug: cytarabine|Drug: idarubicin | City of Hope Medical Center|National Cancer Institute (NCI) | Phase 2 | 1994-09-01 | 2016-12-12 |
| NCT01040559 | Carcinoma, Hepatocellular | Drug: idarubicin | Centre Hospitalier Universitaire Dijon | Phase 1 | 2009-12-01 | 2013-01-08 |
| NCT01289457 | Leukemia | Drug: Clofarabine|Drug: Idarubicin|Drug: Cytarabine|Drug: Fludarabine | M.D. Anderson Cancer Center | Phase 1|Phase 2 | 2011-02-02 | 2017-02-15 |
| NCT01700413 | Di Novo Acute Myeloid Leukemia | Drug: Idarubicin | Grupo Cooperativo de Estudio y Tratamiento de las Leucemias Agudas y Mielodisplasias|Ministry of Health, Spain|Fundaci贸 Institut de Recerca de l'Hospital de la Santa Creu i Sant Pau | Phase 2 | 2012-10-01 | 2016-01-27 |
| NCT01035502 | Acute Myeloid Leukemia | Drug: Elacytarabine plus idarubicin | Clavis Pharma|Theradex|INC Research | Phase 2 | 2009-12-01 | 2013-09-20 |
| NCT00878722 | Acute Myeloid Leukemia | Drug: PXD101|Drug: idarubicin | Onxeo | Phase 1|Phase 2 | 2007-08-01 | 2015-07-06 |
| NCT00382954 | Acute Myelogenous Leukemia | Drug: Velcade|Drug: Idarubicin | University of Kentucky|National Institutes of Health (NIH) | Phase 1 | 2004-02-01 | 2010-09-28 |
| NCT01025154 | Acute Myeloid Leukemia | Drug: Clofarabine|Drug: Idarubicin|Drug: Cytarabine | M.D. Anderson Cancer Center|Genzyme, a Sanofi Company | Phase 2 | 2010-01-01 | 2013-08-19 |
| NCT00656617 | Acute Myeloid Leukemia (AML)|Myelodysplastic Syndrome (MDS) | Drug: Idarubicin|Drug: Cytarabine|Drug: Vorinostat | M.D. Anderson Cancer Center|Merck Sharp & Dohme Corp. | Phase 2 | 2008-04-01 | 2015-02-26 |
| NCT02635074 | Recurrent Adult Acute Myeloid Leukemia | Drug: Cytarabine|Drug: Ibrutinib|Drug: Idarubicin | Bruno C. Medeiros|National Cancer Institute (NCI)|Stanford University | Phase 1 | 2016-11-01 | 2016-12-08 |
| NCT00542971 | AML|Acute Myeloid Leukemia|Myelodysplastic Disorders | Drug: Idarubicin|Drug: Sorafenib|Drug: Ara-C | M.D. Anderson Cancer Center|Bayer | Phase 1|Phase 2 | 2007-10-01 | 2012-06-12 |