-
生物活性
CX-4945 is an ATP-competitive CK2 protein kinase inhibitor with a Ki and an IC50 of 0.38 and 1 nM for recombinant human CK2α, respectively. CX-4945 has broad-spectrum anti-proliferative activity in multiple cancer cell lines. The anti-proliferative activity of CX-4945 against cancer cells correlated with expression levels of the CK2α catalytic subunit. Attenuation of PI3K/Akt signaling by CX-4945 was evidenced by dephosphorylation of Akt on the CK2-specific S129 site and the canonical S473 and T308 regulatory sites. CX-4945 suppresses Akt signaling and inhibits proliferation of HUVEC Cells. CX-4945 causes cell-cycle arrest and selectively induced apoptosis in certain cancer cells. In models of angiogenesis, CX-4945 inhibited human umbilical vein endothelial cell migration, tube formation, and blocked CK2-dependent hypoxia-induced factor 1 alpha (HIF-1α) transcription in cancer cells. Collectively, CX-4945 inhibits pro-angiogenic CK2 signaling in vitro and in vivo. CX-4945 is an inhibitor of Flt-3/Flk-2 and Pim-1.
Kinase activities
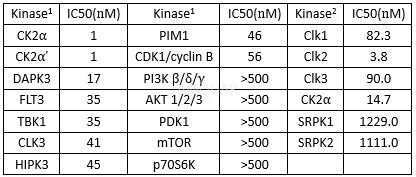
Antiproliferativeactivity of CX2-4945
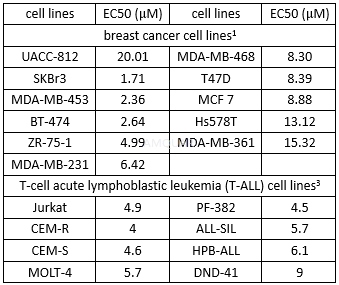
CellAntiproliferative Activity[4]
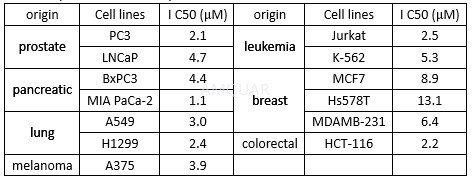
-
体外研究
-
体内研究
-
激酶实验
In vitro kinase assays[2]
Recombinantkinases were incubated with 50 mM HEPES (pH 7.5), 0.01% BRIJ-35, 10 mM MgCl2,1 mM EGTA, and Ser/Thr peptide. After the 1 hour kinase reaction, 5 μL of a 1:512 dilution of Development Reagentsolution was added. The reaction was developed and terminated, and then thefluorescence ratio was calculated according to the manufacturer’s protocol.
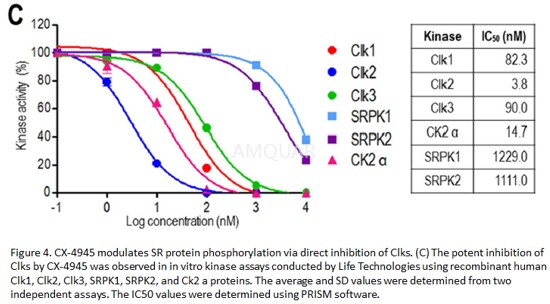
-
细胞实验
Cellcultures and reagents[5]
The human NSCLC cell lines A549, H1299,Calu-1 and H358 were cultured in RPMI-1640 containing 10% fetal bovine serum,100units/ml penicillin, and 100μg/ml streptomycin at 37˚C in an atmosphere with5% CO2.
Cellsurvival assays
Cells (5×105and 1×106)were seeded into a 60mm dish in triplicate and were treated with the respectiveagents for 72 h. Cells were trypsinized and cell numbers were determined usingan ADAM-MC automatic cell counter according to the manufacturer’s instructions.
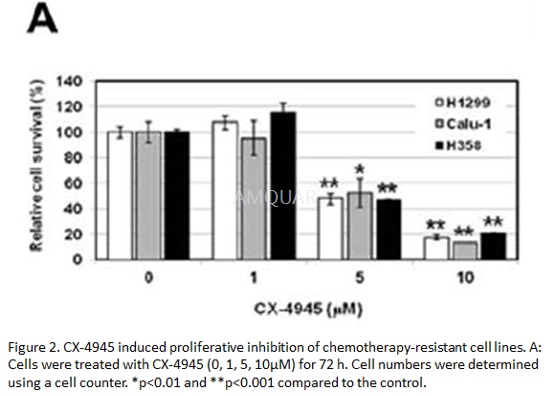
-
动物实验
In vivo subcutaneous human T-ALL mouse model[3]
Mice were housed and bred in a specificpathogen-free animal facility. Eight weeks old nonobese diabetic/severecombined immunodeficient mice were subcutaneously injected in both flanks with10x106MOLT-4.Luc.GFP cells and resuspended in 100μlof phosphate-buffered saline. On day 5, mice were injected with luciferin to assesstumor burden by whole-body bioluminescence imaging and were equally distributedin two groups to receive CX-4945 dissolved in 25mM disodium hydrogen phosphate(Na2HPO4) or vehicle control. Tumor growth was monitoredweekly by bioluminescence and caliper measurements, and mice were weighedfrequently to determine treatment-induced toxicity. For bioluminescenceimaging, mice were anaesthetized, intraperitoneally injected with 150mgD-luciferin per kg body weight and scanned with an IVIS Lumina bioimagingdevice after 15 min. Total flux (photons per second) was calculated using the LivingImage software.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Siddiqui-Jain A, Drygin D, Streiner N, et al. CX-4945, an orally bioavailable selective inhibitor of protein kinase CK2, inhibits prosurvival and angiogenic signaling and exhibits antitumor efficacy. Cancer Res. 2010;70(24):10288-10298.
[2] Kim H, Choi K, Kang H, et al. Identification of a novel function of CX-4945 as a splicing regulator. PLoS One. 2014;9(4):e94978.
[more]
分子式
C19H12ClN3O2 |
分子量
349.77 |
CAS号
1009820-21-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
25 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01199718 | Multiple Myeloma | Drug: CX-4945 | Cylene Pharmaceuticals | Phase 1 | 2010-09-01 | 2011-06-13 |
| NCT02128282 | Cholangiocarcinoma | Drug: CX-4945|Drug: Cisplatin|Drug: Gemcitabine | Senhwa Biosciences, Inc. | Phase 1|Phase 2 | 2014-06-01 | 2016-10-14 |
| NCT00891280 | Advanced Solid Tumors|Breast Cancer|Inflammatory Breast Cancer|Castleman's Disease|Multiple Myeloma | Drug: CX-4945 oral formulation | Cylene Pharmaceuticals | Phase 1 | 2009-02-01 | 2011-06-13 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们