-
生物活性
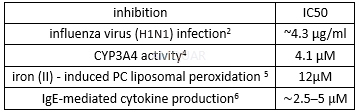
Fluvastatin, Sodium Salt is a synthetic inhibitor of HMGCR (HMG-CoA reductase) (IC50 = 40-100 nM for human liver microsomes) that acts as an anti-hypercholesterolemic compound and as an antioxidant. Fluvastatin also inhibits induction of the LDL receptor on HepG2 cells at a concentration of 0.1-1.0 μM. Research shows that fluvastatin can inhibit the formation of TBA-reactive substances in Fe(II)-supported peroxidation of liposomes (IC50 = 12 μM). Inhibits vascular smooth muscle proliferation in vitro (IC50 = 70 nM) and exhibits antihypercholesterolemic and antioxidant activity in vivo.
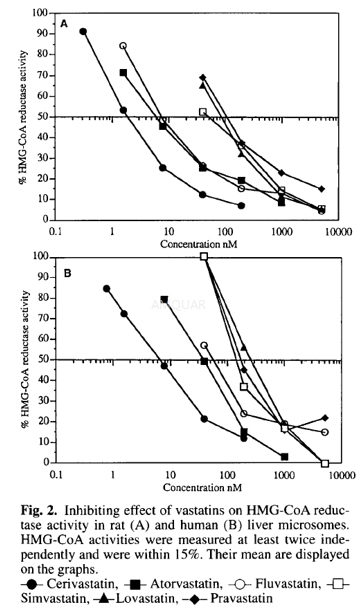
HMG-CoAreductase activity of fluvastatin[1]

Cytotoxicity of fluvastatin

Biological activities of fluvastatin
-
体外研究
-
体内研究
30% propylene glycol, 5% Tween 80, 65% D5W(5%葡萄糖水溶液)
-
激酶实验
HMG-CoA reductase activity[1]
HMG-CoA reductase activitywas measured using modifications of the assay. The total volume ofeach assay was 95μI and the reaction mixture contained 10μI of theinhibiting compound to be tested and 85μI of theincubating buffer containing 2mg/ml liver microsomes, 0.1M KH2P04,pH 7.2, 5.7mM dithiothreitol, 10mM glucose-6-phosphate, 2U/mlglucose-6-phosphate dehydrogenase, 1mM NADP, 10μM miconazole.Control experiments were done without NADPH generating system. All samples wereincubated 10 min at 37°C before addition of 5μI ofsubstrate (unlabelled and14C-HMG-3-hydroxy3- methyl glutaryl CoA,final concentration 50μM, 2.5nCi/nmole). After 30 min at 37OC, the reaction was stoppedby adding 27μI 1N HCl and 20μI of unlabelled mevalonolactone (200μg/assay). Theconversion of mevalonic acid to lactone was performed at room temperature for60 min.
One hundred III of the assay wereapplicated to a 2ml column containing 100-200 mesh AG 1X8 CI form. Mevalonolactonewas eluted with 4 x 500μI of H2O. The eluates were mixed with 6 ml of Pico-Fluor40. The radioactivity of the samples was counted in a Packard Tricarbscintillation counter with automatic quenching correction. IC50 values wereobtained by plotting the percentage of activity against test compound concentration.
The same experiment done with an internalstandard (3H-mevalonolactone) has indicated that the percentage of recoverywas 80% ± 10%. Control experiment checking the formation of mevalonolactone wasrealized spotting directly40μI of the incubation mixture onto Silica Gel thinlayer plates and thechromatogram was developed in acetone:cyclohexane (1: 1, vol./vol.). Theradioactivity profile was analysed and quantified using a radioactive TLCscanner.
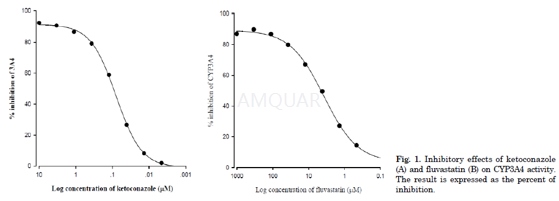
CYP inhibition assay[4]
The assays of inhibition of human CYP3A4enzyme activity were performed in a multiwell plate using the CYP inhibition assaykit. Briefly, human CYP enzymes were obtained from baculovirus-infected insectcells. CYP substrate (7-BFC) was incubated with or without fluvastatin inenzyme/substrate buffer consisting of 1 pM of P450 enzyme and a NADPHgenerating system (1.3 mM NADP, 3.54 mM glucose 6-phosphate, 0.4 U/ml glucose6-phosphate dehydrogenase and 3.3 mM MgCl2) in potassium phosphatebuffer (pH 7.4). Reactions were terminated by adding stop solution after 45-minincubation. Metabolite concentrations were measured by spectrofluorometer at anexcitation wavelength of 409 nm and an emission wavelength of 530 nm. Positivecontrol (1 μM ketoconazole) was run on the same plate and produced99% inhibition. Results are expressed as the percent of inhibition.
-
细胞实验
Cells[2]
Madin‑Darby canine kidney(MDCK) cells and a human epithelial lung cell line (A549) were grown in minimumessential medium (MEM) and Dulbecco's Modified Eagle medium/Nutrient MixtureF12 (DMEM/F12), respectively, with 10% heat‑inactivatedfetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100μg/ml).
Cytotoxicitytest and IC50 determination
MDCK or A549 cells were seeded onto 96‑well plates (5x105cells/well) and incubated for 24 h.Following treatment with MEM containing two‑foldserially diluted fluvastatin for 48 h, the medium was removed and 50 μl MTT wasadded into each well and incubated for 3 h at 37˚C, 5% CO2. Dimethylsulphoxide (DMSO; 150 μl/well) was then added, and the absorbance of the cellswas determined at 490 nm. The 50% cytotoxic concentration (CC50) wascalculated. Anti‑influenza virus activity of fluvastatinwas analysed and the 50% inhibition concentration (IC50) was determined. MDCKcells were infected with H1N1 and a multiplicity of infection (MOI) of 0.1 for1 h. Maintenance medium containing serial two‑folddilutions of fluvastatin (0‑20μg/ml) was added and thecells were then incubated for 48 h at 37˚C, 5% CO2. The MTT assay wasperformed as described above. Oseltamivir (0‑4μg/ml; ) was used as thepositive control.
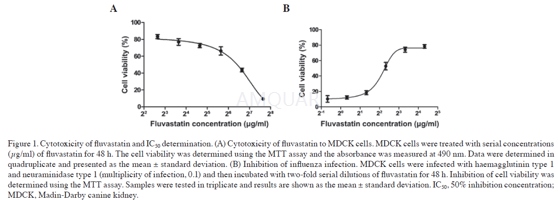
-
动物实验
Passivesystemic anaphylaxis[6]
C57BL/6J and 129/SvImJ mice, at a minimumof 10 wk old, were administered 200μl PBS containing 1 mgfluvastatin or equivalent dilution of DMSO via i.p. injection, followed by 200μlPBS containing 50μg mouse IgE anti-DNP. The following day, mice were againadministered 200μl PBS containing 1 mg fluvastatin, or DMSO 1 h before DNP-HSA (50μg)was administered via i.p. injection. In some experiments, 8 mg histamine wasinjected in place of Ag. The core body temperature of each mouse was measuredusing a rectal microprobe. Mice were euthanized and blood was collected bycardiac puncture to analyze plasma.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Dansette PM, Jaoen M, Pons C. HMG-CoA reductase activity in human liver microsomes: comparative inhibition by statins. Experimental and Toxicologic Pathology. 2000;52(2):145-148.
[more]
分子式
C24H25FNNaO4 |
分子量
433.45 |
CAS号
93957-55-2 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
25 mM |
Ethanol
25 mM |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01551173 | Lipid Metabolism Disorders | Drug: Fluvastatin sodium | Novartis Pharmaceuticals|Novartis | Phase 4 | 2012-01-01 | 2015-08-10 |
| NCT00664742 | Metabolic Syndrome | Drug: Fluvastatin XL庐 | Novartis | Phase 4 | 2006-09-01 | 2011-04-19 |
| NCT00814723 | Hypercholesterolemia | Drug: fluvastatin|Drug: Fluvastatin plus ezetimibe | Medical University of Graz | Phase 4 | 2005-09-01 | 2008-12-24 |
| NCT00752843 | Healthy Subjects | Drug: mifepristone + fluvastatin | Corcept Therapeutics | Phase 1 | 2008-09-01 | 2012-02-15 |
| NCT00674297 | Antiphospholipid Syndrome | Drug: Fluvastatin | Hospital for Special Surgery, New York|University of Texas | Phase 2 | 2008-05-01 | 2013-02-03 |
| NCT00421005 | Heart Transplantation|Hypercholesterolemia | Drug: fluvastatin | University of Bologna | Phase 4 | 2004-11-01 | 2009-04-28 |
| NCT00487318 | Chronic Hepatitis C | Drug: standard of care|Drug: fluvastatin | Bader, Ted, M.D.|VA Office of Research and Development | Phase 2 | 2007-06-01 | 2012-08-20 |
| NCT00565474 | Graft Vasculopathy | Drug: Fluvastatin | Novartis Pharmaceuticals|Novartis | Phase 4 | 2001-09-01 | 2017-02-21 |
| NCT01681199 | Coronary Heart Disease|Atherosclerosis | Drug: Fluvastatin extended release tablet | Beijing Anzhen Hospital|Novartis | Phase 4 | 2012-07-01 | 2012-09-05 |
| NCT00814606 | Hepatitis C|Hepatitis C Virus | Drug: Fluvastatin|Drug: Peginterferon alfa2a|Drug: ribavirin | University of Chicago | Phase 2 | 2010-02-01 | 2013-06-28 |
| NCT00138528 | Metabolic Syndrome | Drug: Fluvastatin | Novartis | Phase 4 | 2004-10-01 | 2017-02-20 |
| NCT00441493 | Hepatitis C | Drug: fluvastatin | Bader, Ted, M.D.|VA Office of Research and Development | | 2006-09-01 | 2012-07-26 |
| NCT00416403 | Breast Cancer | Drug: fluvastatin sodium|Procedure: Breast Cancer Surgery Only - Arm III | University of California, San Francisco|National Cancer Institute (NCI) | Phase 2 | 2006-07-01 | 2012-12-12 |
| NCT00171262 | Dyslipidemia | Drug: Fluvastatin | Novartis | Phase 4 | 2004-08-01 | 2017-02-20 |
| NCT00382161 | Impotence | Drug: Fluvastatin-sodium | University Hospital, Saarland|Novartis | Phase 3 | 2006-10-01 | 2009-02-12 |
| NCT00404287 | Aortic Valve Stenosis | Drug: Fluvastatin | AORTICA Group | Phase 4 | 2006-10-01 | 2014-10-13 |
| NCT00223041 | Renal Transplantation | Drug: Fluvastatin | University of Schleswig-Holstein | Phase 2|Phase 3 | 2003-04-01 | 2011-08-04 |
| NCT00136799 | Mixed Dyslipidemia|Hypercholesterolemia | Drug: Fluvastatin | Novartis | Phase 3 | 2005-06-01 | 2017-02-20 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们