-
生物活性
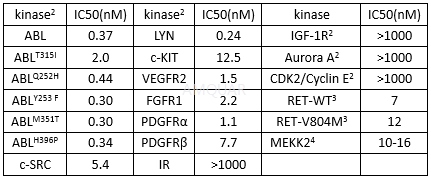
Ponatinib(AP24534), a potent non‑selective TKI, which targets BCR‑ABL, FGFR, VEGFR and PDGFR, has been approved by the FDA for treatingimatinib-resistant patients with chronic myelogenous leukemia, including thoseharboring the gatekeeper mutant T315I Bcr-Abl. AP 24534 is a small molecule, multi-target kinase inhibitor known to potently inhibit Bcr-Abl (the fusion protein of Bcr and c-Abl), Flk-1(VEGFR2) and FGFR. It also exhibits inhibitory activity against PDGFRα, c-Src and c-Kit. AP 24534 inhibits the proliferation of mutant FLT3-positive cells (IC50 of 13 nM), which inhibits mutant FLT3 phosphorylation (IC50 of 1 nM) and the proliferation of Bcr-Abl1 T315I-positive Ba/F3 cells with an IC50 of 8 nM. Displays potent activity against cell lines expressing native Bcr-Abl or Bcr-AblT3151 (IC50 values are 0.37 and 2.0 nM respectively); also inhibits other Abl kinase domain mutants at nanomolar potencies. Exhibits inhibitory activity against PDGFRα, c-Src and c-Kit (IC50 values are 1.1, 5.4 and 12.5 nM respectively); potently inhibits FGFR and VEGFR family kinases.
Ponatinib potently inhibits [125I]-IAAPbinding to ABCG2 and ABCB1 with IC50 values of 0.04μM and 0.63μM, respectively.[1]
KinaseInibition Profile of AP24534
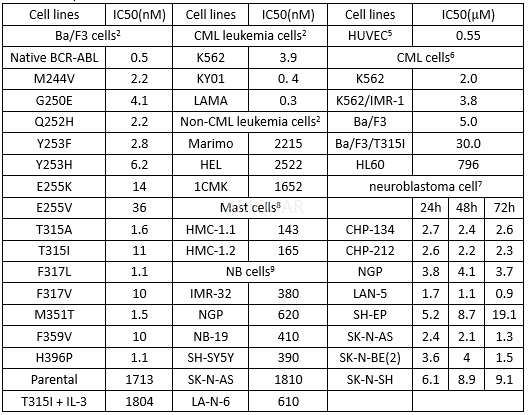
Cell viability
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% Propylene glycol
-
激酶实验
Invitro kinase assay[10]
Subconfluent cells were solubilized inlysis buffer without phosphatase inhibitors. Then, 50g of proteins wereimmunoprecipitated with anti-RET; immunocomplexes were recovered with protein Asepharose beads, washed 5 times with kinase buffer, and incubated 20 min at roomtemperature in kinase buffer containing 2.5μCi[γ-32P]ATPand unlabelled ATP to a final concentration of 20μM. Sampleswere separated by SDS-PAGE. Gels were dried and exposed to autoradiography.Signal intensity was analyzed by the Phosphorimager interfaced with theImageQuant software. Half maximal kinase inhibitory concentration (IC50) valuewas calculated by using PRISM (GraphPad software).
-
细胞实验
HUVECsculture[5]
HUVECs were cultured in endothelial culturegrowth medium (ECGM) supplemented with ECGM Supplement Mix. Unless indicatedotherwise, experiments were performed in M199 medium supplemented with10% fetalbovine serum (FBS), 50μg/mL endothelial cell growth supplement,5IU/mL heparin, 2mML-glutamine, 100 U/mL penicillin, and 0.1 mg/ml streptomycin. For allexperiments, HUVECs passages <5 were used.
Isolationof EPCs
Human early EPCs (eEPCs) were isolated frombuffy coats donated by healthy volunteers. Peripheral blood mononuclear cells (PBMCs)were isolated by Ficoll density-gradient centrifugation. A total of 1x106or 135,000 PBMCs were placed on fibronectin-coated 24-cm culture dishes or96-well plates, respectively. PBMCs were maintained for 7 days in complete M199medium. After 7 days, these PBMCs formed a typical cell cluster surrounded byspindle-shaped cells. These cells were considered eEPCs. Endothelial lineagewas confirmed by VE-cadherin (PE-labeled) immunostaining.
Cellviability assay
Viability was measured by tetrazolium WST-1assay. Briefly, HUVECs were plated in 96-well plates and incubated in thepresence of imatinib, nilotinib, and ponatinib. After 48 hours, 10μLWST-1 reagent was added to the plate and incubated for 1 hour at37OC,at which point the absorbance (450 nm) was measured in an ELISA plate reader.For EPC and macrophage viability, 4–5 days after plating the cells were treatedwith 0.17μM ponatinib or DMSO. Three days (for EPCs) and 4–5 days (formacrophages) after treatment, cell viability was assessed using WST-1 reagent,as detailed above.
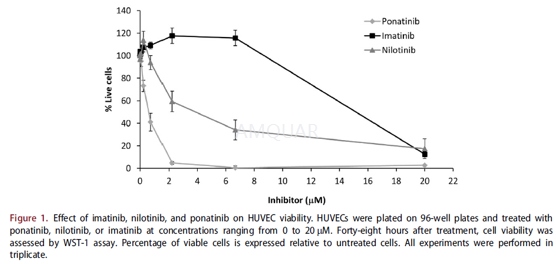
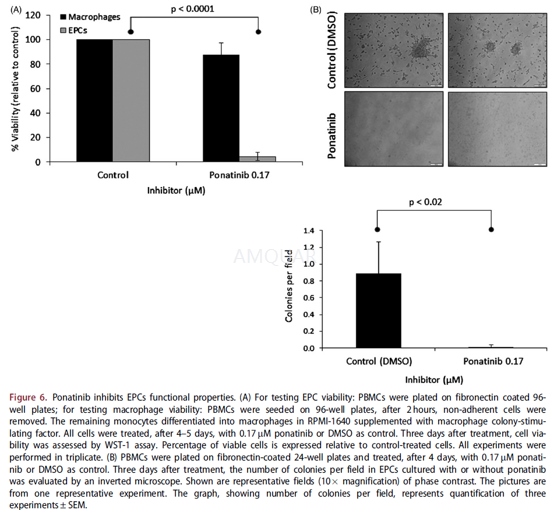
-
动物实验
Orthotopic mouse model of NB[9]
Five to six-week-old female athymic NCRnude mice were maintained under barrier conditions (pathogen free conditionsprovided by plastic cages with sealed air filters). As described previously, thepreclinical mouse model of NB was established using orthotopic (intrarenal) implantationof the NB cells. A transverse incision was created over the left flank of thenude mouse, and 1.0 x106human luciferase-transduced NGP cellssuspended in0.1 ml of PBS were surgically injected into the left renal capsuleand towards the superior pole of the left kidney of the nude mice. Afterallowing the cells to engraft for two to three weeks, mice bearing tumors ofsimilar sizes (using bioluminescent imaging to monitor tumor growth) were randomizedinto treatment with DMSO or ponatinib (15 mg/kg/day) for 21 days. At the end ofthe treatment, all mice were dissected. Tumors and the right kidneys (control)were harvested and weighed. Another set of in vivo experiments were performedfor the protein immunoblotting assay. Four weeks after implantation,NGP-luciferase cell xenografted mice were selected and treated with either DMSOor ponatinib via i.p. injection (15 mg/kg) once daily for two days. Tumors werethen harvested for the protein immunoblotting assay.
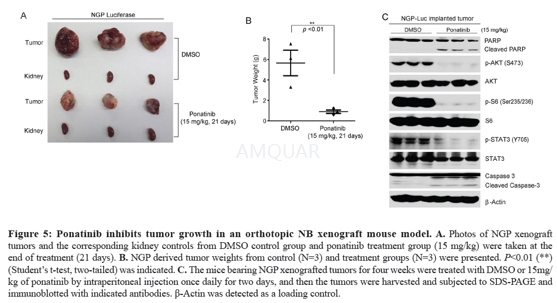
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Sen R, Natarajan K, Bhullar J, et al. The novel BCR-ABL and FLT3 inhibitor ponatinib is a potent inhibitor of the MDR-associated ATP-binding cassette transporter ABCG2. Mol Cancer Ther. 2012;11(9):2033-2044.
[2] O'Hare T SW, Zhu X, Eide CA, Rivera VM, Wang F, Adrian LT, Zhou T, Huang WS, Xu Q, Metcalf CA 3rd, Tyner JW, Loriaux MM, Corbin AS, Wardwell S, Ning Y, Keats JA. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. . 2009;16(5):401-412.
[3] Mologni L, Redaelli S, Morandi A, Plaza-Menacho I, Gambacorti-Passerini C. Ponatinib is a potent inhibitor of wild-type and drug-resistant gatekeeper mutant RET kinase. Mol Cell Endocrinol. 2013;377(1-2):1-6.
[4] Ahmad S, Johnson GL, Scott JE. Identification of ponatinib and other known kinase inhibitors with potent MEKK2 inhibitory activity. Biochem Biophys Res Commun. 2015;463(4):888-893.
[more]
分子式
C29H27F3N6O |
分子量
532.56 |
CAS号
943319-70-8 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
<1 mg/mL |
Ethanol
50 mM |
体内溶解度
约10.5 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02428543 | Acute Myeloid Lukemia | Drug: Ponatinib and Cytarabine | Versailles Hospital | Phase 1|Phase 2 | 2013-07-01 | 2016-08-19 |
| NCT01746836 | Leukemia | Drug: Ponatinib | M.D. Anderson Cancer Center|Ariad Pharmaceuticals | Phase 2 | 2013-01-01 | 2016-11-16 |
| NCT01761747 | Non-Small Cell Lung Cancer, Head and Neck Cancer | Drug: ponatinib | Dana-Farber Cancer Institute | Phase 2|Phase 3 | 2013-01-01 | 2014-12-13 |
| NCT01888562 | Endometrial Neoplasms | Drug: Ponatinib | Washington University School of Medicine | | 2016-01-01 | 2015-12-17 |
| NCT01592136 | Chronic Myeloid Leukemia (CML)|Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ ALL) | Drug: ponatinib | Ariad Pharmaceuticals | | null | 2013-03-12 |
| NCT01935336 | Adenocarcinoma of the Lung|Extensive Stage Small Cell Lung Cancer|Limited Stage Small Cell Lung Cancer|Recurrent Non-small Cell Lung Cancer|Recurrent Small Cell Lung Cancer|Stage IIIA Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer | Drug: Ponatinib | University of Colorado, Denver|Ariad Pharmaceuticals | Phase 2 | 2013-09-01 | 2016-11-07 |
| NCT01570868 | Leukemia | Drug: Ponatinib | M.D. Anderson Cancer Center|Ariad Pharmaceuticals | Phase 2 | 2012-04-01 | 2016-05-13 |
| NCT01667133 | Chronic Myeloid Leukemia (CML)|Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia (Ph+ ALL) | Drug: ponatinib - Phase 1|Drug: ponatinib - Phase 2 | Ariad Pharmaceuticals | Phase 1|Phase 2 | 2012-08-01 | 2014-08-26 |
| NCT02478164 | Glioblastoma | Drug: Ponatinib | Dana-Farber Cancer Institute | Phase 2 | 2015-07-01 | 2016-10-11 |
| NCT02627677 | Chronic Phase Chronic Myeloid Leukemia | Drug: ponatinib 30 mg QD|Drug: ponatinib 15 mg QD|Drug: nilotinib 400 mg BID | Ariad Pharmaceuticals | Phase 3 | 2015-12-01 | 2015-12-09 |
| NCT01813734 | Non Small Cell Lung Cancer | Drug: Ponatinib | Massachusetts General Hospital | Phase 2 | 2013-09-01 | 2016-09-12 |
| NCT01549548 | Philadelphia Chromosome Positive (Ph+) Leukemias|Chronic Myeloid Leukemia | Drug: Ponatinib | OHSU Knight Cancer Institute|Ariad Pharmaceuticals | | null | 2013-03-13 |
| NCT02398825 | Chronic Myeloid Leukemia|Chronic Phase|Adults | Drug: Ponatinib | Gruppo Italiano Malattie EMatologiche dell'Adulto | Phase 2 | 2016-08-01 | 2016-09-14 |
| NCT02272998 | Malignant Neoplasm | Drug: ponatinib hydrochloride|Other: laboratory biomarker analysis | Sameek Roychowdhury|Ohio State University Comprehensive Cancer Center | Phase 2 | 2015-02-01 | 2016-05-06 |
| NCT01207440 | Chronic Myeloid Leukemia|Ph+ Acute Lymphoblastic Leukemia | Drug: Ponatinib | Ariad Pharmaceuticals | Phase 2 | 2010-09-01 | 2016-10-28 |
| NCT01641107 | Philadelphia Positive|BCR-ABL Positive|Acute Lymphoblastic Leukemia | Drug: Ponatinib | Gruppo Italiano Malattie EMatologiche dell'Adulto | Phase 2 | 2014-10-01 | 2016-09-14 |
| NCT02467270 | Myeloid Leukemia, Chronic, Chronic Phase | Drug: ponatinib 45 mg|Drug: ponatinib 30 mg|Drug: ponatinib 15 mg | Ariad Pharmaceuticals | Phase 2 | 2015-06-01 | 2017-03-02 |
| NCT02829840 | Leukemia|FLT3-Mutated Acute Myeloid Leukemia|FLT3-Mutated High-Risk Myelodysplastic Syndrome | Drug: Ponatinib|Drug: 5-azacytidine|Behavioral: Phone Calls | M.D. Anderson Cancer Center|Ariad Pharmaceuticals | Phase 1|Phase 2 | 2016-09-01 | 2016-08-05 |
| NCT02265341 | Malignant Hepatobiliary Neoplasm | Other: Laboratory Biomarker Analysis|Drug: Ponatinib Hydrochloride|Other: Quality-of-Life Assessment | Mayo Clinic|National Cancer Institute (NCI) | Phase 2 | 2014-12-01 | 2016-12-20 |
| NCT01874665 | GIST | Drug: ponatinib | Ariad Pharmaceuticals | Phase 2 | 2013-06-01 | 2016-10-27 |
| NCT01838642 | Thyroid Neoplasms | Drug: Ponatinib | National Institutes of Health Clinical Center (CC)|National Cancer Institute (NCI) | Phase 2 | 2013-03-01 | 2016-12-30 |
| NCT02981784 | Leukemia | Drug: Ponatinib (Iclusig庐)|Procedure: allogeneic stem cell transplantation | Hospices Civils de Lyon | | 2000-01-01 | 2016-12-01 |
| NCT00660920 | Chronic Myelogenous Leukemia|Hematologic Malignancies | Drug: Ponatinib | Ariad Pharmaceuticals | Phase 1 | 2008-05-01 | 2016-02-12 |
| NCT01650805 | Chronic Myeloid Leukemia | Drug: ponatinib|Drug: imatinib (Gleevec/ Glivec) | Ariad Pharmaceuticals | Phase 3 | 2012-06-01 | 2014-11-05 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们