-
生物活性
Estrogen receptor antagonist/partial agonist. Selective and potent inhibitor of mammalian sterol isomerase. Neuroprotective in female rats in vivo. Also high affinity agonist at the membrane estrogen receptor GPER.
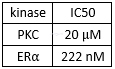
Kinaseinhibition of tamoxifen[1]
Bindingactivity of tamoxifen for hERα and hERβ in the presence of 10 nM estrogen[2]

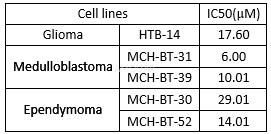
Cytotoxicityof tamoxifen in pediatric brain tumor cells[3]
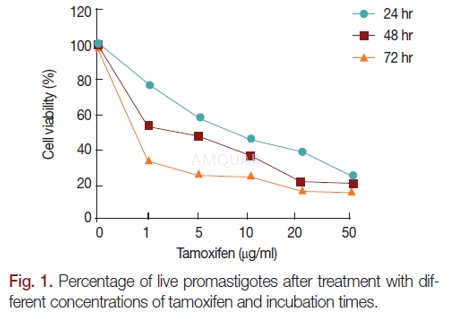
Theefficacy of tamoxifen on leishmaniasis[4]

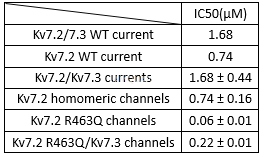
Inhibitionof tamoxifen for Kv7.2/Kv7.3 channels[5]
Tamoxifenstimulates phosphoinositide kinase activity with an ED50 of 20 μM, in the presenceof vanadate.[5]
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% Propylene glycol
-
激酶实验
Estrogen receptor binding assay[6]
Apparent affinities to the compounds for theestrogen receptor were determined by competition binding with [3H]estradiol (E2) to the rat uterine cytosol receptor. Incubations were performedat 25°C for 3 h and nonspecific binding determined using an excess (1000 nM) ofradioinert estradiol. The apparent affinities were expressed as relativebinding affinity (RBA) or in % of displacement at a concentration of 10μM.Calculations were performed according to the following equations: (1) RBA = 100× IC50 of E2/ICs0 of tested compound, where IC50 is the concentration of E 2 ortested compound that inhibits [3H]E2 binding by 50%, the RBA of E2being taken as 100%; and (2) % of displacement at 10μM=100×[(A-B)/(A-C)], where: A is [3H]E2 bound (without tested compound), Bis [3H]E2 bound (with 10/μM of tested compound), C is [3H]E2bound (with 1μM unlabeled E 2); the% of E2 being taken as 100%.
PKC assay[1] To evaluate the ability of tamoxifen andthe triarylacrylonitrile analogues to inhibit PKC activity, SHSY5Y cells wereincubated in Krebs Ringer HEPES (KRH) buffer (25 mM HEPES, 125 mM NaCl, 4.8 mMKCl, 1.2 mM KH2PO4, 1.3 mM CaCl2, 1.2 mM MgSO4, pH7.4) at 37OC for15 min followed by a 1 h treatment with 3 and10μM of eachcompound in KRH. PKC was activated by adding a final concentration of 333 nMphorbol 12-myristate 13-acetate (PMA) in KRH to the samples for 15 min and thereaction quenched with 1 mL cold KRH. The samples were pelleted at 3000 rpmfor2 min. The pellets were washed twice in cold KRH and lysed in solubilizationbuffer (1% Triton X-100, 50 mM Tris HCl, 150 mM NaCl, pH 7.4). Lysates wererotated at 4OC for 1 h and centrifuged at14000 rpm for 15 min toremove debris. Protein assays were conducted using the Biorad DC Protein AssayKit. PKC activity was quantified using Western Blot analysis.
-
细胞实验
Parasiteand culture[4]
This study was carried out with Iranian L.major strain (MRHO/IR/75/ER). Leishmania promastigotes were cultured inRPMI-1640 medium with penicillin (100 units/ml), streptomycin (100 μg/ml), and20% FBS (fetal bovine serum). A hundred μl of media containing 2×105promastigoteswere cultured in each well of 96-well plates and treated with 100 μl ofdifferent concentrations of tamoxifen (1, 5, 10, 20, and 50 μg/ml). Numbers ofparasites were counted at 24, 48, and 72 hr of drug treatment. All the resultsare the average of triplicate experiments. Glucantime was used as positivecontrol drug. IC50 of tamoxifen and glucantime were calculated by GraphPadPrism 5 software.
Determiningthe viability of promastigotes by MTT Assay
A hundred μl of culture media (RPMI-1640with 20% FBS) containing 105promastigotes were added to each wellof 96-well plates. A hundred μl of different concentrations of tamoxifen orglucantime was added to designate wells. Two hundred μl of media were added to3 wells as negative controls. After 24, 48, and 72 hr incubation, 20 μl of MTTreagent (5 mg/ml) was added to each well and incubated for 4 hr at 24˚C in dark.The plate was centrifuged at 3,000 rpm for 10 min. Then, the supernatant wasremoved from wells, and 100 μl DMSO (dimethyl sulfoxide) was added to eachwell. The optical density of the wells was read at 450 nm by an ELISA reader.The following formula was used to calculate the cell viability percent: Cellviability=[AT-AB]/[AC-AB] ×100, where AT is the optical density of wells withcells treated with the drug, AB is the optical density of blank wells, and ACis the optical density of control wells.
-
动物实验
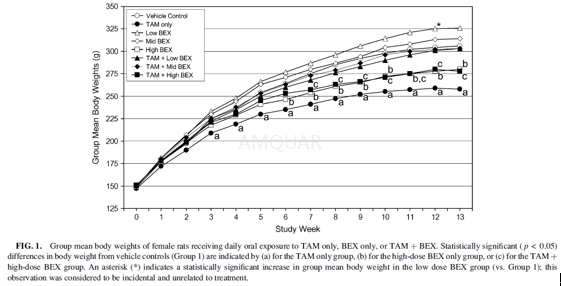
Animals[7]
Female CD IGS rats [Crl:CD(SD)],approximately 4 weeks of age upon receipt, were purchased. During a 14-dayquarantine period, rats were assigned to experimental groups using arandomization process designed to ensure a comparable initial mean body weightin each group. Rats were individually housed in stainless steel cages suspendedover absorbent cage boards, and were held in a temperature-controlled roommaintained on a 12-h light/12-h dark cycle. Rats were also allowed free accessto Certified Rodent Diet 5002 throughout the study, except during an overnightfast prior to scheduled necropsies. Animals were observed a minimum of twicedaily to monitor their general health status. Detailed clinical examinationsand measurements of body weight and food consumption were performed onceweekly.
Experimentaldesign
Female rats (20/group) received daily oral(gavage) exposure totamoxifen (TAM) (in 0.5% ethanol in corn oil) at doses of 0or 60μg/kg/day and/or bexarotene (BEX) (in corn oil) at doses of 0, 5, 15,or 45 mg/kg/day for a minimum of 90 days using a dosing volume of 5 ml/kg bodyweight. After the completion of the dosing period, surviving rats wereeuthanized and necropsied approximately 24 h after the final dose of TAM and/orBEX.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Carpenter C, Sorenson RJ, Jin Y, et al. Design and synthesis of triarylacrylonitrile analogues of tamoxifen with improved binding selectivity to protein kinase C. Bioorg Med Chem. 2016;24(21):5495-5504.
[2] Zou A MK, Arnold KE, Berger EM, Fitzgerald P, Mais DE, Allegretto EA. Estrogen receptor beta activates the human retinoic acid receptor alpha-1 promoter in response to tamoxifen and other estrogen receptor antagonists, but not in response to estrogen. Mol Endocrinol. 1999 13(3):418-430.
[3] Ramachandran C KZ, Petkarou A, Fort J, Fonseca HB, Melnick SJ, Escalon E. Tamoxifen modulation of etoposide cytotoxicity involves inhibition of protein kinase C activity and insulin-like growth factor II expression in brain tumor cells. J Neurooncol. . 2004;67(1-2):19-28.
[more]
分子式
C32H37NO8 |
分子量
563.64 |
CAS号
54965-24-1 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
<1 mg/mL |
Ethanol
5 mM |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02513849 | Cancer|Gastrointestinal Neoplasms | Drug: Tamoxifen | University of Nottingham | Phase 1 | 2015-08-01 | 2015-07-30 |
| NCT02197897 | Bladder Cancer | Drug: Tamoxifen Citrate | Baylor College of Medicine|National Cancer Institute (NCI) | Phase 2 | 2015-06-01 | 2016-10-04 |
| NCT00749138 | Chronic Hepatitis C | Drug: tamoxifen | Bader, Ted, M.D. | Phase 1 | 2008-11-01 | 2010-03-07 |
| NCT01027416 | Breast Cancer | Drug: Tamoxifen | Roswell Park Cancer Institute|National Institutes of Health (NIH)|National Cancer Institute (NCI) | | 2009-12-14 | 2017-02-07 |
| NCT01196936 | Breast Cancer | Drug: Tamoxifen Citrate|Drug: Placebo|Procedure: Digital mammography|Other: immunohistochemistry staining method|Other: pharmacological study|Other: laboratory biomarker analysis|Genetic: protein expression analysis|Other: pharmacogenomic studies|Other: questionnaire administration|Procedure: Fine needle aspiration|Other: Quality of Life Assessment | University of Alabama at Birmingham|National Cancer Institute (NCI)|St. Jude Children's Research Hospital|University Health Network, Toronto|University of Michigan|Dana-Farber Cancer Institute|Mayo Clinic|University of Washington|City of Hope National Medical Center|M.D. Anderson Cancer Center|University of Chicago|University of Minnesota - Clinical and Translational Science Institute|University of Colorado, Denver|Wake Forest University | Phase 2 | 2010-09-01 | 2016-11-30 |
| NCT01181518 | Poisoning by, Adverse Effect of and Underdosing of Tamoxifen | | Inje University | | 2010-08-01 | 2013-05-31 |
| NCT02311647 | Endometrial Receptivity Post Tamoxifen Exposure | | Sheba Medical Center | | 2015-01-01 | 2014-12-22 |
| NCT01192308 | Breast Cancer | Drug: Tamoxifen 40mg QD | Radboud University | Phase 1 | 2010-07-01 | 2012-05-21 |
| NCT00532454 | Breast Cancer|Metastatic Disease | Drug: Tamoxifen | National Cancer Center, Korea | Phase 2 | 2006-06-01 | 2010-02-09 |
| NCT02089386 | Barrett Metaplasia | Drug: Tamoxifen|Procedure: Endoscopy | Washington University School of Medicine | Early Phase 1 | 2014-07-09 | 2017-03-09 |
| NCT02824224 | Menorrhagia|Metrorrhagia|Medicated Intrauterine Devices | Drug: Tamoxifen|Drug: Placebo (for Tamoxifen) | Oregon Health and Science University | Phase 4 | 2016-09-01 | 2017-02-03 |
| NCT02166944 | Amyotrophic Lateral Sclerosis|ALS Functional Ration Scale|TAR-DNA-binding Protein-43|Tamoxifen|mTOR | Drug: tamoxifen 40 mg daily for one year | Taipei Medical University Shuang Ho Hospital | Phase 1|Phase 2 | 2014-04-01 | 2014-06-16 |
| NCT02668666 | Hormone Receptor Positive Malignant Neoplasm of Breast|Human Epidermal Growth Factor 2 Negative Carcinoma of Breast|Estrogen Receptor Positive Breast Cancer|Progesterone Receptor Positive Tumor|Metastatic Breast Cancer | Drug: Palbociclib|Drug: Tamoxifen | Oana Danciu, MD|Pfizer|Hoosier Cancer Research Network|Big Ten Cancer Research Consortium | Phase 2 | 2016-06-01 | 2017-02-06 |
| NCT00963209 | Breast Cancer | Drug: tamoxifen citrate|Other: laboratory biomarker analysis|Other: pharmacological study | Centre Hospitalier Universitaire Vaudois | Phase 3 | 2009-06-01 | 2013-07-31 |
| NCT00165308 | Hodgkin's Disease|Breast Cancer | Drug: Tamoxifen | Dana-Farber Cancer Institute|Brigham and Women's Hospital|Massachusetts General Hospital|AstraZeneca | | 2001-04-01 | 2014-07-08 |
| NCT00309491 | Early-stage Breast Cancer | Drug: Tamoxifen alone|Drug: Tamoxifen + Aminoglutethimide | Austrian Breast & Colorectal Cancer Study Group | Phase 3 | 1990-12-01 | 2016-04-08 |
| NCT01257581 | Amyotrophic Lateral Sclerosis | Drug: creatine|Drug: tamoxifen | Nazem Atassi|ALS Therapy Alliance|State University of New York - Upstate Medical University|Massachusetts General Hospital | Phase 2 | 2011-03-01 | 2014-12-03 |
| NCT00710970 | Urinary Bladder Neoplasms | Drug: Tamoxifen | Seth Lerner|AstraZeneca|Cytogen Corporation|Baylor College of Medicine | Phase 2 | 2007-01-01 | 2013-07-22 |
| NCT01075802 | Breast Cancer|CYP2D6 Polymorphism | Drug: Tamoxifen | Western Sydney Local Health District|St George Hospital, Australia | | 2010-03-01 | 2013-05-03 |
| NCT00411203 | Bipolar Disorder | Drug: Tamoxifen Citrate|Drug: Placebo | Stanley Medical Research Institute | Phase 3 | 2003-04-01 | 2014-07-30 |
| NCT02322853 | Postmenopausal|Metastatic Breast Cancer | Drug: Tamoxifen|Drug: Ralimetinib (LY2228820 dimesylate) | Centre Francois Baclesse|National Cancer Institute, France|ARC Foundation for Cancer Research | Phase 2 | 2015-01-01 | 2016-06-22 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们