-
生物活性
Tranylcypromine HCl is an irreversible inhibitor of lysine-specific demethylase 1 (LSD1/BHC110) and monoamine oxidase (MAO). IT inhibits histone demethylation. In combination with CHIR 99021, enables reprogramming of mouse embryonic fibroblasts transduced by only two factors, Oct4 and Klf4, into induced pluripotent stem (iPS) cells.
Inhibitionof tranylcypromine (tPCPA) · HCl for LSD and MAO enzymes[1]

Inhibition of tranylcypromine (TCP) for thereuptake of norepinephrine

MAOinhibition of tranylcypromine in tissue homogenates[4]

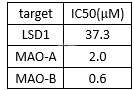
Inhibitionof tranylcypromine for LSD and MAO enzymes[5]
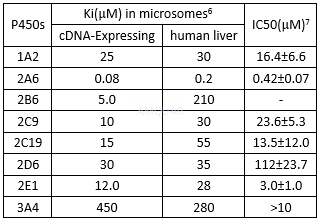
Inhibition of tranylcypromine (TCP) for P450sin human liver microsomes[6]

Theinhibition of tranylcypromine against human CYP isoforms[6]
-
体外研究
-
体内研究
-
激酶实验
Determinationof MAO activity[4]
MAO assay was performed utilizing themeasurement of deaminated14C-metabolites of14C-labelledMAO substrates. In a typical protocol 10μl enzymepreparation [homogenates containing 1- 5μg protein in0.1mol/L phosphate buffer (pH 7.4)], 10μl 0.1mol/Lphosphate buffer containing the MAO inhibitors in the respective experimentsand 20μl MAO substrate in 0.1mol/L phosphate buffer labelled with 100μmol/L5-[2-14C]hydroxytryptamine (1.6Ci/mol, 15,000cpm), 100μmol/L[1-14C]tyramine (1.6Ci/mol, 15,000cpm) or 10μmol/L 2-[l-14C]phenylethylamine(12.6Ci/mol, 15,000cpm) were mixed in a glass-stoppered centrifuge tube andincubated for 20 min at 37oC. In control experiments the reactionwas found to be linear up to 30 min of incubation. In experiments for the determinationof the km-values concentrations of the labeled substrates (15,000cpm) andaccordingly of the specific activities were varied as described in the resultssection. The reaction was stopped by addition of 100μl 2N HCl and deaminated14C-metabolites were extracted into 6ml toluene-ethylacetate (1:1).After centrifugation, a 4 ml aliquot of the organic layer was transferred to avial containing 5 ml scintillation liquid and counted in a liquid scintillationspectrophotometer. The counting efficiency was 95.6%. Specific activity wasexpressed as nmol substrate deaminated per mg protein and per min.

-
细胞实验
Cellculture[8]
Human prostate carcinoma cells (LNCaP-LN3)were grown in MEM, supplemented with 10% fetal bovine serum, penicillin (100U/ml)/streptomycin (100μg/ml) at 37˚C in a 5% CO2atmosphere.
Cellproliferation assay
The proliferation of LNCaP-LN3 cells wasevaluated using a Premix WST-1 Cell Proliferation Assay System. After exposingthe cells to pargyline or tranylcypromine (0.5, 1, 1.5 or 2mM) for 24, 48, 72,96 or 120 h, the culture medium was removed and the cells were washed withphosphate buffered saline (PBS). WST-1 reagent was then added, and the cells wereincubated for 4 h. The results of the WST-1 assay were measured using a Model680 microplate reader.

-
动物实验
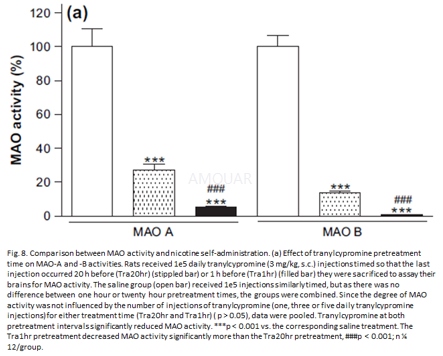
Animaltreatments[9]
Adult rats received one, three or fivedaily tranylcypromine (3 mg/kg, i.p.) injections timed so that the lastinjection occurred 20 h or 1 h prior to sacrifice and removal of their brains.Saline groups received one, three or five injections (1.5 ml/kg, i.p.)similarly timed, but with no difference between pretreatment times, they werecombined. The number of injections was chosen according to behavioral data:rats began to prefer the reinforced hole on day 3 and this effect wassignificant on day 5.
Tissuepreparation
Male rats were decapitated, their brainsremoved immediately and homogenized in 8 ml of 50mM sodium phosphate buffer(PB), pH 7.4. Homogenates were centrifuged at 600xg for 10min at 4oC, and the resulting supernatants were diluted with PB to afinal tissue concentration of 20 mg/ml. Samples (100 ml) were taken foranalysis of protein content using a Bradford protein assay. The remainingtissue samples were used for enzymatic analysis of MAO-A and -B activitiesusing a radiochemical assay, slightly modified from established procedures. Briefly,100 ml tissue aliquot was incubated in a reaction volume of 1 ml PB buffer,pH7.4, with [14C]serotonin (5-HT)
or [14C]phenylethylamine (PEA)for measurement of MAO-A and MAO-B activities, respectively. Radioligands weresupplemented with addition of unlabeled ligand to yield a final concentrationof 1 mM within the assay mixture. Blanks were prepared in the same manner but omittingbrain homogenate. After incubation for 20 min at 37oC, reactionswere terminated by cooling on ice and addition of 100 ml of 6N HCl. Thedeaminated products were extracted from the assay mixture by the addition of 6ml of benzene/ethyl acetate for [14C]5-HTand 6 ml of toluene for [14C]PEAand vortexed for 30 s. Tubes were centrifuged for 7 min at 1400 x g to separatethe organic and inorganic phases. Four ml of the organic layer was removed andadded to 5 ml of Safety-Solve scintillation fluid for scintillation counting.Data were reported as pmol/mg protein/min.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Binda C VS, Romanenghi M, Pilotto S, Cirilli R, Karytinos A, Ciossani G, Botrugno OA, Forneris F, Tardugno M, Edmondson DE, Minucci S, Mattevi A, Mai A. Biochemical, structural, and biological evaluation of tranylcypromine derivatives as inhibitors of histone demethylases LSD1 and LSD2. J Am Chem Soc. . 2010 132(19):6827-6833.
[2] Horn AS SS. Steric requirements for catecholamine uptake by rat brain synaptosomes: studies with rigid analogs of amphetamine. J Pharmacol Exp Ther. . 1972;180(3):523-530.
[3] Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM. The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology. 2011;61(1-2):95-104.
[more]
分子式
C9H12ClN |
分子量
169.65 |
CAS号
1986-47-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mM |
Water
100 mM |
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT01430455 | Bipolar Disorder I or II | Drug: Tranylcypromine | New York State Psychiatric Institute | Phase 4 | 2011-05-01 | 2011-12-14 |
| NCT00653393 | To Determine the Bioavailability of Tranylcypromine | Drug: Tranylcypromine|Drug: Parnate | Par Pharmaceutical, Inc.|SFBC Ft. Myers, Inc | Phase 1 | 2004-10-01 | 2008-04-03 |
| NCT00296686 | Major Depression | Drug: Tranylcypromine|Drug: Dextroamphetamine|Drug: Triiodothyronine | New York State Psychiatric Institute | Phase 4 | 2001-09-01 | 2012-04-26 |
| NCT02717884 | Acute Myeloid Leukemia|Myelodysplastic Syndrome | Drug: tranylcypromine|Drug: all-trans retinoic acid|Drug: cytarabine | Ulrike Kohlweyer|University Hospital Freiburg | Phase 1|Phase 2 | 2015-05-01 | 2016-06-01 |
| NCT01031810 | Major Depressive Disorder | Drug: tranylcypromine | New York State Psychiatric Institute|National Institute of Mental Health (NIMH) | Phase 4 | 2009-11-01 | 2014-09-15 |
| NCT02273102 | Acute Myelogenous Leukemia|Myelodysplastic Syndromes|Leukemia | Drug: Tranylcypromine|Drug: Tretinoin | University of Miami | Phase 1 | 2015-03-02 | 2017-03-07 |
| NCT03044184 | Stroke Hemorrhagic|Intracerebral Haemorrhage | Drug: Tranexamic Acid | Kwong Wah Hospital | Phase 3 | 2017-04-01 | 2017-02-04 |
| NCT01643135 | Bleeding | Drug: tranexamic acid|Drug: 0.9% NaCl | Mahidol University | Phase 4 | 2012-06-01 | 2013-12-17 |
| NCT00612807 | Major Depressive Disorder|Partner Relational Disorder (V61.10) | Behavioral: Weekly marital therapy|Drug: As indicated: Sertraline, bupropion, venlafaxine, mirtazepine, nortriptyline, tranylcypromine, lithium augmentation, etc. | Duke University|National Institutes of Health (NIH) | Phase 1|Phase 2 | 2006-07-01 | 2013-06-17 |
| NCT00223691 | Autonomic Failure|Orthostatic Hypotension | Drug: Atomoxetine|Drug: Acarbose|Drug: Pyridostigmine Bromide|Drug: Yohimbine|Drug: Midodrine HCl|Drug: placebo|Drug: Modafinil|Drug: Octreotide|Other: water intake|Drug: Diphenhydramine Hydrochloride|Drug: Ranitidine HCL|Drug: Tranylcypromine|Drug: Ergotamine/ Caffeine|Drug: Celecoxib|Drug: Pseudoephedrine|Drug: Methylphenidate|Drug: Indomethacin|Drug: Ibuprofen|Drug: Oxymetazoline 0.05% nasal solution|Dietary Supplement: Bovril|Drug: Acetazolamide|Drug: Rivastigmine tartrate|Drug: Carbidopa/levodopa|Device: Inflatable abdominal binder|Device: inflatable abdominal binder (sham) | Vanderbilt University|Vanderbilt University Medical Center | Phase 1 | 2002-03-01 | 2017-01-16 |
| NCT01896349 | Treatment Resistant Depression | Other: IPT+ antidepressant drugs|Drug: fluoxetine|Drug: sertraline|Drug: paroxetine|Drug: Citalopram|Drug: escitalopram|Drug: fluvoxamine|Drug: Venlafaxine|Drug: Duloxetine|Drug: Bupropion|Drug: Lithium|Drug: Risperidone|Drug: tranylcypromine|Drug: Imipramine|Drug: amitriptyline|Drug: Clomipramine|Drug: nortriptyline|Drug: trazodone|Drug: Mirtazapine|Drug: sulpiride | Hospital de Clinicas de Porto Alegre | | 2013-04-01 | 2013-07-10 |
| NCT02458729 | Osteoarthritis, Knee | Drug: Tranexamic Acid 5%,5ml/amp (intraoperative)|Drug: Tranexamic Acid 5%,5ml/amp (3 hours after operation)|Drug: 0.9% Normal Saline (intraoperative)|Drug: 0.9% Normal Saline (3 hours after operation)|Drug: rivaroxaban (10mg) | Chang Gung Memorial Hospital | Phase 4 | 2012-08-01 | 2015-05-28 |
| NCT02453802 | Osteoarthritis, Knee | Drug: Tranexamic Acid 5%,5ml/amp|Drug: Tranexamic Acid 5%,5ml/amp|Drug: rivaroxaban (10mg)|Drug: 0.9% Normal Saline|Drug: 0.9% Normal Saline | Chang Gung Memorial Hospital | Phase 4 | 2015-06-01 | 2015-05-26 |
| NCT00994994 | Blood Loss|Congenital Heart Disease | Drug: Tranexamic Acid | Okayama University | | 2006-01-01 | 2009-10-13 |
| NCT02865174 | Osteoarthritis, Knee | Drug: Topical tranexamic acid|Drug: Floseal庐|Drug: Enoxaparin | Chang Gung Memorial Hospital | Phase 4 | 2016-09-01 | 2016-08-09 |
| NCT00704860 | Major Depression | Other: Open label pharmacotherapy | University of Ottawa | Phase 4 | 2005-02-01 | 2011-01-18 |
| NCT01713101 | Acute Upper Gastrointestinal Hemorrhage | Drug: Early intravenous tranexamic acid administration|Drug: placebo | Seoul National University Hospital | Phase 3 | 2012-10-01 | 2014-05-14 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们


























