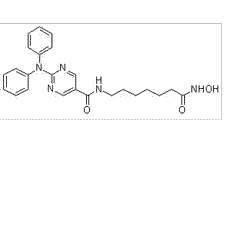
-
生物活性
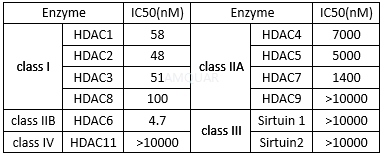
Rocilinostat is a selective HDAC6 inhibitor with IC50 of 5 nM >10-fold more selective for HDAC6 than HDAC1/2/3 (class I HDACs) with slight activity against HDAC8, minimal activity against HDAC4/5/7/9/11, Sirtuin1, and Sirtuin2. Rocilinostat has minimal activity (IC50 > 1μM) against HDAC4, HDAC5, HDAC7, HDAC9, HDAC11, Sirtuin1, and Sirtuin2, and has slight activity against HDAC8 (IC50 = 0.1μM). The IC50 values for Rocilinostat for T-cell toxicity is 2.5μM. Rocilinostat overcomes tumor cell growth and survival conferred by BMSCs and cytokines in the BM milieu. Rocilinostat in combination with bortezomib induces synergistic anti-MM activity. Rocilinostat induces potent acetylation of α-tubulin at very low doses and triggers acetylation of lysine on histone H3 and histone H4 only at higher doses, confirming its specific inhibitory effect on HDAC6 activity.
Inhibitionof HDAC enzymes[1]
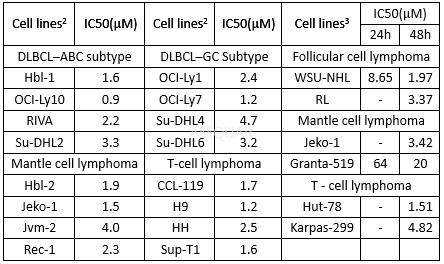
Growth inhibition of ACY-1215 on lymphomacell lines
-
体外研究
-
体内研究
1% DMSO+30% polyethylene glycol+1% Tween 80
-
激酶实验
HDACenzymatic assays[1]
ACY-1215 was dissolved and subsequentlydiluted in assay buffer [50mM HEPES, pH 7.4, 100mM KCl, 0.001% Tween-20, 0.05%BSA, and 20μM tris(2-carboxyethyl)phosphine] to 6-fold the final concentration. HDACenzymes were diluted to 1.5-fold of the final concentration in assay buffer andpre-incubated with ACY-1215 for 10 minutes before the addition of thesubstrate. The amount of FTS (HDAC1, HDAC2, HDAC3, and HDAC6) or MAZ-1675(HDAC4, HDAC5, HDAC7, HDAC8, and HDAC9) used for each enzyme was equal to theMichaelis constant (Km), as determined by a titration curve. FTS or MAZ-1675was diluted in assay buffer to 6-fold the final concentration with 0.3μMsequencing grade trypsin. The substrate/trypsin mix was added to the enzyme/compoundmix and the plate was shaken for 60 seconds and then placed into a SpectraMaxM5 microtiter plate reader. The enzymatic reaction was monitored for release of7-amino-4-methoxy-coumarin over 30 minutes, after deacetylation of the lysineside chain in the peptide substrate, and the linear rate of the reaction wascalculated. HDAC11, sirtuin1, and sirtuin2 assays were performed by Cerep.
-
细胞实验
Reagents and cells culture[3]
Reagents were dissolved in DMSO and storedat −20 °C until use. In all experiments, the final concentration of DMSO whichwas used as vehicle control did not exceed 0.01%. Ricolinostat was investigatedusing a panel of six NHL cell lines: WSU-NHL, RL (follicular lymphoma,FL),Granta-519, Jeko-1 (mantle cell lymphoma, MCL), Hut-78 (cutaneous T celllymphoma, CTCL) and Karpas-299 (anaplastic large cell lymphoma, ALCL). With theexception of GRANTA-519, lymphoma cell lines were cultured in RPMI-1640supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 U/mLpenicillin and streptomycin. For Granta-519 cells, DMEM was used in place ofRPMI-1640. Cell lines used in this study were thawed from early passage stocksand were passaged for less than 6 months.
Viabilityassay and clonogenic formation
Cell viability was evaluated by MTTcolorimetric assay following the manufacturer’s instructions. NHL cell lineswere incubated in triplicate with increasing concentrations of ricolinostat (0.01–100μM)and bendamustine (25–300μM) as single agents for 24–72 h to identify the IC50 valuesof each drug.
For assessment of drug combination effect,serial dilutions of the two agents were assessed using concentrations lower thanthe IC50. NHL cell lines were cultured with fixed doses of ricolinostat (2,2.5, 4, 5, 8, 10μM) and bendamustine (10, 20, 25, 40, 50, 100 μM).
For clonogenic assays, NHL cell lines werefirst exposed to ricolinostat alone and in combination with bendamustine inliquid culture for 24–48 h, then collected and incubated in methylcellulose andmaintained for 10 or 14 days. Growing colonies (>50 cells) were countedunder a microscope.
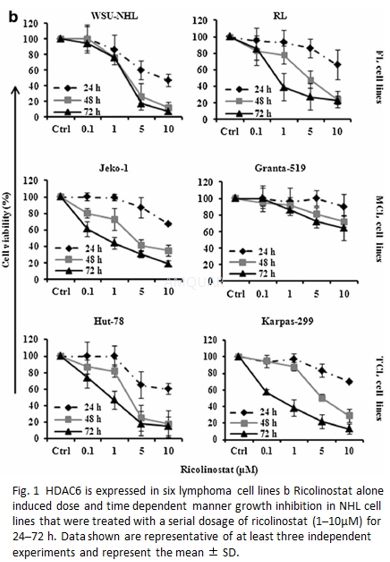
-
动物实验
In vivo studies[4]
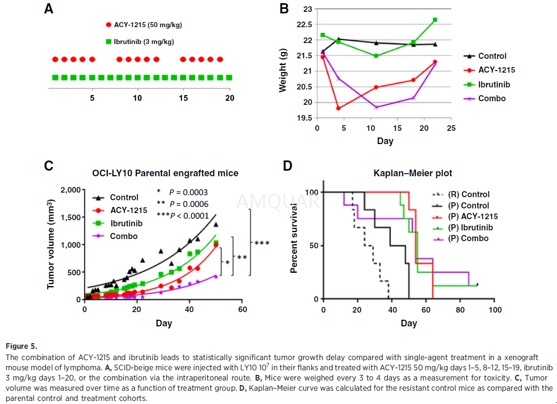
Animals were housed and maintained inaccordance with an IUCAC-approved protocol. OCI-Ly10 cells (1x107;either resistant or parental) in 50% Matrigel were subcutaneously injected intothe flanks of 5- to 7-week-old beige/SCID mice. Treatment was initiated whentumor volume measured 80mm3. Tumor volume was assessed using the twolargest perpendicular axes (l= length; w = width) and calculated using theformula v = 0.5(l2x w). Mice were divided into 4 cohorts of 8–10mice per cohort as follows: (i) untreated control; (ii) ACY-1215: 50 mg/kg days1–5, 8–12, 15–19; (iii) ibrutinib: 3 mg/kg days 1–20; (iv) ACY-1215 plusibrutinib. Drugs were diluted in sterile dextrose5% in water and wereadministered via the intraperitoneal (IP) route. Mice were assessed for weightloss and tumor volume 3x/week. Animals were sacrificed when the tumor volumeexceeded 2,000 mm3or after sustained loss of >10% body weight inaccordance with institutional guidelines.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Santo L, Hideshima T, Kung AL, et al. Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood. 2012;119(11):2579-2589.
[2] Amengual JE, Johannet P, Lombardo M, et al. Dual Targeting of Protein Degradation Pathways with the Selective HDAC6 Inhibitor ACY-1215 and Bortezomib Is Synergistic in Lymphoma. Clin Cancer Res. 2015;21(20):4663-4675.
[more]
分子式
C24H27N5O3 |
分子量
433.5 |
CAS号
1316214-52-4 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
85 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01323751 | Multiple Myeloma | Drug: ACY-1215 | Acetylon Pharmaceuticals Incorporated|The Leukemia and Lymphoma Society | Phase 1|Phase 2 | 2011-07-01 | 2017-01-23 |
| NCT01997840 | Multiple Myeloma | Drug: ACY-1215 (Ricolinostat) in combination with pomalidomide and dexamethasone | Acetylon Pharmaceuticals Incorporated | Phase 1|Phase 2 | 2013-11-01 | 2016-10-17 |
| NCT02091063 | Lymphoma|Lymphoid Malignancies | Drug: ACY-1215 | Jennifer Amengual|Acetylon Pharmaceuticals Incorporated|Columbia University | Phase 1|Phase 2 | 2014-04-02 | 2017-02-14 |
| NCT02632071 | Metastatic Breast Cancer|Breast Carcinoma | Drug: ACY-1215|Drug: Nab-paclitaxel | Kevin Kalinsky|Acetylon Pharmaceuticals Incorporated|Columbia University | Phase 1 | 2016-02-01 | 2016-10-13 |
| NCT01583283 | Multiple Myeloma | Drug: ACY-1215|Drug: lenalidomide|Drug: Dexamethasone | Acetylon Pharmaceuticals Incorporated | Phase 1 | 2012-04-01 | 2016-10-12 |
| NCT02189343 | Multiple Myeloma | Drug: ACY-1215 in combination with pomalidomide and dexamethasone | Acetylon Pharmaceuticals Incorporated | Phase 1 | 2014-08-01 | 2016-10-14 |
| NCT02787369 | Recurrent Chronic Lymphoid Leukemia | Drug: ACY-1215|Drug: Ibrutinib|Drug: Idelalisib | Dana-Farber Cancer Institute|Acetylon Pharmaceuticals Incorporated | Phase 1 | 2016-05-01 | 2016-11-16 |
| NCT02088398 | Healthy | Drug: ACY-1215 | Acetylon Pharmaceuticals Incorporated | Phase 1 | 2014-03-01 | 2014-09-10 |
| NCT02856568 | Non-Resectable Cholangiocarcinoma|Recurrent Cholangiocarcinoma|Stage III Extrahepatic Bile Duct Cancer|Stage III Intrahepatic Cholangiocarcinoma|Stage IIIA Hilar Cholangiocarcinoma|Stage IIIB Hilar Cholangiocarcinoma|Stage IVA Extrahepatic Bile Duct Cancer|Stage IVA Hilar Cholangiocarcinoma|Stage IVA Intrahepatic Cholangiocarcinoma|Stage IVB Extrahepatic Bile Duct Cancer|Stage IVB Hilar Cholangiocarcinoma|Stage IVB Intrahepatic Cholangiocarcinoma|Unresectable Extrahepatic Bile Duct Carcinoma | Drug: Cisplatin|Drug: Gemcitabine Hydrochloride|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Drug: Ricolinostat | Mayo Clinic|National Cancer Institute (NCI) | Phase 1 | 2016-10-01 | 2016-08-02 |
| NCT02661815 | Ovarian Cancer|Fallopian Tube Cancer|Primary Peritoneal Carcinoma | Drug: Paclitaxel|Drug: Ricolinostat|Drug: Bevacizumab | Dana-Farber Cancer Institute|Acetylon Pharmaceuticals Incorporated | Phase 1 | 2016-01-01 | 2017-02-27 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们