-
生物活性
Ganciclovir inhibits the replication of viruses including CMV, Herpes Simplex, Varicella Zoster and Epstein-Barr. It inhibits virus replication by its incorporation into viral DNA. This incorporation inhibits dATP and leads to defective DNA, ceasing or retarding the viral machinery required to spread the virus to other cells.
Ganciclovirinduces DNA damage (increases the number of γ-H2AX foci) with an IC50 of 0.05μM.[1]
Antiviralactivity of ganciclovir in HCMV-infected U373 cellsand MRC-5 fibroblasts.[2]
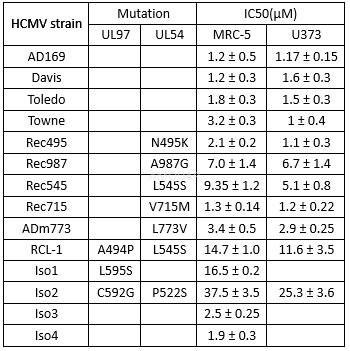
Antiviral effect of ganciclovir
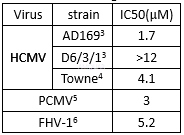
Antiviralefficacy against herpes simplex virus type 1(HSV-1)[7] 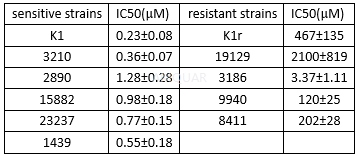
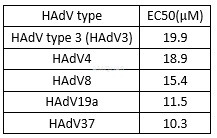
Ganciclovirinhibits HAdV proliferation with CC50 of 212μg/mL (827μM).[8]Antiviralactivity of ganciclovir against human adenovirus (HAdV)[8]
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
cells[8]
A549 cells (alveolar epithelial cells) werecultured in Eagle’s Minimum Essential Medium (MEM) containing 2 mM l-glutamine,0.1 mM nonessential amino acids, and 7% fetal calf serum.
Viruses
HAdV type 3 (HAdV3), HAdV4, HAdV8, HAdV19,and HAdV37 were used. HAdV3, 4, 8, and 37 were prototype strains. Since HAdV19p(prototype strain) has never induced keratoconjunctivitis, we used a clinical strain,HAdV19a, for this study. These strains were propagated in A549 cells and storedat -80°C until use.
Cytotoxicityassay
The cytotoxicity of ganciclovir wasevaluated in A549 cells. Dilutions of ganciclovir were prepared in Eagle’sMinimum Essential Medium supplemented with 2% fetal calf serum. The medium wasthen discarded and replaced for 24 hours by medium containing eightconcentrations of ganciclovir: 62.5, 125, 250, 500, 1,000, 2,000, 4,000, and8,000μg/mL. After 7 days’ incubation at 37°C with 5% CO2, thecells in the plates were then subjected to a3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium(MTS)-based colorimetric assay for cell viability according to themanufacturer’s instructions. A490 values, corrected for the cytotoxicityexerted by ganciclovir (as determined in mock-infected cultures), were used tocalculate the percentage of cell viability. The 50% cytotoxic concentration(CC50) was determined as the value causing destruction of 50% of the monolayercells, by regression analysis.

-
动物实验
Mice[9]
C57BL/6 and FvB mice were between 8 and12wk of age when experiments were initiated.
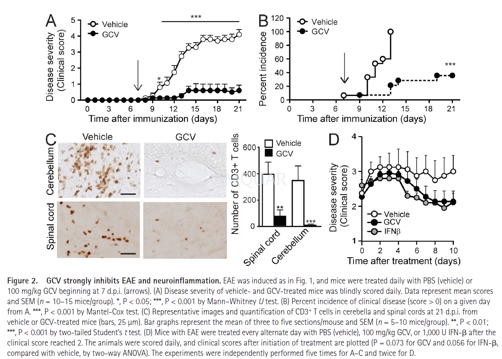
EAEinduction and assessment
MOG35–55peptide(MEVGWYRSPFSRVVH-LYRNGK) was synthesized and purified to >95% purity. Mice(8–12wk old) were immunized subcutaneously with a total of 200μg MOG35–55peptide emulsified in CFA (200μg mycobacterium tuberculosis) and received ani.v. injection of 400ng pertussis toxin at the time of immunization and 48 hlater. Mice were weighed and examined daily for clinical signs of EAE andscored: 0, no paralysis; 1, loss of tail tone; 2, hind limb weakness orparesis; 3, hind limb paralysis; 4, hind limb paralysis and forelimb paresis;5, moribund or dead.
50mg/kg BrdU was injected intraperitoneallyfor 3 d before sacrifice to label dividing cells.
Kainicacid administration
Kainic acid was dissolved in PBS andinjected subcutaneously (20mg/kg) in mice. Mice were then weighed and examineddaily for clinical signs of seizures.
GCV,IFN-β, andmitoxantrone treatments
Clinical grade GCV/ Ganciclovir wasobtained as a lyophilized powder and reconstituted in deionized water beforeuse. The drug was administered daily via intraperitoneal injections at 25 or100mg/kg body weight. In some experiments, 100mg/kg GCV, 1,000U/dose ofrecombinant mouse IFN-βor PBS was given intraperitoneally every other day after the micereached a clinical score of 2 or more. 0.5 mg/kg mitoxantrone was administeredintraperitoneally daily. Mice were scored daily starting at 7 d.p.i.
Tissuepreparation
Mice were anesthetized with 400 mg/kgchloral hydrate before removal of the spleen, which was dissected in halves andweighed. Mice were then transcardially perfused with 0.9% saline, followed bythe removal of the spinal cord and brain, which were dissected into twohemibrains. One half of the spleen, a hemibrain and the entire spinal cord werefixed for 24 h in 4% paraformaldehyde and cryoprotected in 30% sucrose. Brainswere sectioned sagittally at 40 μm, whereas the spleens were sectionedtransversally at 40μm using a freezing microtome and stored in cryoprotectivemedium at 20oC. The other half of brain and spleen were thenprocessed for cell isolation.
Cellisolation from spleen and CNS tissue
Spleens and brains were dissociated bygrinding through 100-μM cell strainers and suspended in RPMI-1640 with 10% FBS (RP10)or HBSS, respectively. To obtain a single-cell suspension, dissociated braincells in HBSS were digested with 300U/ml clostridial collagenase and 50μg/mlDNaseI for 1h at 37°C with mild agitation and halted with the addition of RP10.The brain cells were then washed with HBSS and resuspended in 30% Percoll, followedby an underlay of 70% Percoll and overlay of 10% Percoll. This Percoll gradientwas then centrifuged at 500 g for 20 min at 4°C with the brakes off. Myelindebris was removed from the 10% Percoll layer, and mononuclear cells were collectedfrom the 30%/70% interface and resuspended with RP10 for further flow cytometryanalysis.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Ladd B, O'Konek JJ, Ostruszka LJ, Shewach DS. Unrepairable DNA double-strand breaks initiate cytotoxicity with HSV-TK/ganciclovir. Cancer Gene Ther. 2011;18(10):751-759.
[2] Schnepf N, Corvo J, Pors MJ, Mazeron MC. Antiviral activity of ganciclovir and artesunate towards human cytomegalovirus in astrocytoma cells. Antiviral Res. 2011;89(2):186-188.
[more]
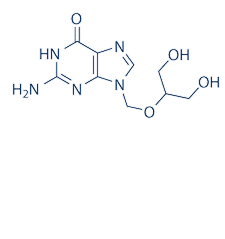
分子式
C9H13N5O4 |
分子量
255.23 |
CAS号
82410-32-0 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
60 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01647529 | Cytomegalovirus Anterior Segment Infection|Anterior Uveitis|Endotheliitis | Drug: Ganciclovir | Singapore National Eye Centre | | 2012-07-01 | 2015-11-16 |
| NCT02943057 | Cytomegalovirus Infections | Drug: 2% guttae ganciclovir | Singapore National Eye Centre | Phase 4 | 2016-08-01 | 2016-10-21 |
| NCT01185223 | Allogeneic Stem Cell Transplantation | Drug: Valganciclovir|Drug: Ganciclovir | Pierrel Research Europe GmbH|Roche Pharma AG | Phase 3 | 2010-09-01 | 2012-12-07 |
| NCT00093704 | Lymphoma|Lymphoproliferative Disorder | Drug: bortezomib + ganciclovir | Jonsson Comprehensive Cancer Center|National Cancer Institute (NCI) | Phase 1 | 2005-03-01 | 2015-10-01 |
| NCT01446445 | Infection in Solid Organ Transplant Recipients | Drug: Ganciclovir/ Valganciclovir according to SPC|Drug: Ganciclovir/ Valganciclovir according to PK model | Nuria Lloberas|Ministerio de Sanidad, Servicios Sociales e Igualdad|Hospital Universitari de Bellvitge | Phase 4 | 2011-12-01 | 2015-03-25 |
| NCT00730769 | Cytomegalovirus Infection | Drug: Single arm (ganciclovir and valganciclovir) | Salvador Gil-Vernet|Roche Pharma AG|Hospital Universitari de Bellvitge | Phase 4 | 2004-03-01 | 2011-09-19 |
| NCT00000143 | Cytomegalovirus Retinitis|HIV Infections | Device: Ganciclovir implant and oral ganciclovir|Drug: Cidofovir intravenous | Johns Hopkins Bloomberg School of Public Health | Phase 3 | 1997-05-01 | 2016-02-18 |
| NCT00241345 | Cytomegalovirus Infections | Drug: Valganciclovir|Drug: Ganciclovir | Washington University School of Medicine | Phase 3 | 2004-06-01 | 2013-07-22 |
| NCT01533480 | Keratoconjunctivitis Due to Adenovirus|Viral Shedding | Drug: Zirgan|Drug: genteal gel | Lifelong Vision Foundation|Bausch & Lomb Incorporated | Phase 4 | 2012-01-01 | 2015-03-30 |
| NCT01335932 | Acute Lung Injury|Acute Respiratory Distress Syndrome|Respiratory Failure | Drug: IV Ganciclovir|Drug: Placebo | Fred Hutchinson Cancer Research Center|National Heart, Lung, and Blood Institute (NHLBI)|Genentech, Inc. | Phase 2 | 2011-09-01 | 2016-07-21 |
| NCT00000805 | Cytomegalovirus Infections|HIV Infections | Drug: Ganciclovir | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 1 | null | 2012-03-30 |
| NCT00000668 | Cytomegalovirus Retinitis|HIV Infections | Drug: Ganciclovir | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 1 | null | 2012-03-15 |
| NCT00000688 | Cytomegalovirus Retinitis|HIV Infections | Drug: Ganciclovir | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 3 | null | 2011-03-11 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们