| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01164995 | Epithelial Ovarian Cancer | Drug: MK-1775 and carboplatin | The Netherlands Cancer Institute|Merck Sharp & Dohme Corp. | Phase 2 | 2010-07-01 | 2012-09-11 |
| NCT02095132 | Childhood Central Nervous System Neoplasm|Recurrent Childhood Medulloblastoma|Recurrent Childhood Supratentorial Embryonal Tumor, Not Otherwise Specified|Recurrent Malignant Solid Neoplasm|Recurrent Neuroblastoma | Drug: Irinotecan Hydrochloride|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 1|Phase 2 | 2014-03-01 | 2017-03-13 |
| NCT01748825 | Neoplasms|Lymphoma | Drug: MK-1775 (AZD1775) | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1 | 2012-11-14 | 2017-02-18 |
| NCT01047007 | Solid Tumors | Drug: MK1775|Drug: Comparator: MK1775 in combination with 5-FU|Drug: Comparator: MK1775 in combination with 5-FU/CDDP | Merck Sharp & Dohme Corp. | Phase 1 | 2010-01-01 | 2015-02-03 |
| NCT01357161 | Ovarian Cancer | Drug: MK-1775|Drug: Placebo|Drug: paclitaxel|Drug: carboplatin | Merck Sharp & Dohme Corp. | Phase 2 | 2011-07-01 | 2016-11-24 |
| NCT02196168 | Recurrent Hypopharyngeal Squamous Cell Carcinoma|Recurrent Laryngeal Squamous Cell Carcinoma|Recurrent Laryngeal Verrucous Carcinoma|Recurrent Lip and Oral Cavity Squamous Cell Carcinoma|Recurrent Metastatic Squamous Cell Carcinoma in the Neck With Occult Primary|Recurrent Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma|Recurrent Oral Cavity Verrucous Carcinoma|Recurrent Oropharyngeal Squamous Cell Carcinoma|Squamous Cell Carcinoma Metastatic in the Neck With Occult Primary|Stage IV Hypopharyngeal Squamous Cell Carcinoma|Stage IVA Laryngeal Squamous Cell Carcinoma|Stage IVA Laryngeal Verrucous Carcinoma|Stage IVA Lip and Oral Cavity Squamous Cell Carcinoma|Stage IVA Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma|Stage IVA Oral Cavity Verrucous Carcinoma|Stage IVA Oropharyngeal Squamous Cell Carcinoma|Stage IVB Laryngeal Squamous Cell Carcinoma|Stage IVB Laryngeal Verrucous Carcinoma|Stage IVB Lip and Oral Cavity Squamous Cell Carcinoma|Stage IVB Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma|Stage IVB Oral Cavity Verrucous Carcinoma|Stage IVB Oropharyngeal Squamous Cell Carcinoma|Stage IVC Laryngeal Squamous Cell Carcinoma|Stage IVC Laryngeal Verrucous Carcinoma|Stage IVC Lip and Oral Cavity Squamous Cell Carcinoma|Stage IVC Nasal Cavity and Paranasal Sinus Squamous Cell Carcinoma|Stage IVC Oral Cavity Verrucous Carcinoma|Stage IVC Oropharyngeal Squamous Cell Carcinoma|Tongue Carcinoma | Drug: Cisplatin|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Other: Placebo|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 2 | 2014-03-01 | 2016-08-04 |
| NCT01076400 | Cervical Cancer | Drug: MK1775 + topotecan + cisplatin|Drug: Comparator: MK1775 + topotecan + cisplatin|Drug: Comparator: Placebo to MK1775 + topotecan + cisplatin | Merck Sharp & Dohme Corp. | Phase 1|Phase 2 | 2010-05-01 | 2015-02-03 |
| NCT02101775 | Ovarian Brenner Tumor|Ovarian Carcinosarcoma|Ovarian Clear Cell Cystadenocarcinoma|Ovarian Endometrioid Adenocarcinoma|Ovarian Mucinous Cystadenocarcinoma|Ovarian Seromucinous Carcinoma|Ovarian Serous Cystadenocarcinoma|Ovarian Serous Surface Papillary Adenocarcinoma|Recurrent Fallopian Tube Carcinoma|Recurrent Ovarian Carcinoma|Recurrent Primary Peritoneal Carcinoma|Undifferentiated Ovarian Carcinoma | Drug: Gemcitabine Hydrochloride|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Other: Placebo|Other: Questionnaire Administration|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 2 | 2014-07-01 | 2017-01-31 |
| NCT00648648 | Solid Tumors | Drug: MK1775|Drug: gemcitabine|Drug: cisplatin|Drug: carboplatin|Drug: MK1775|Drug: MK1775|Drug: MK1775|Drug: gemcitabine|Drug: cisplatin|Drug: carboplatin | Merck Sharp & Dohme Corp. | Phase 1 | 2008-02-01 | 2015-02-03 |
| NCT01958658 | Stage IB Cervical Cancer|Stage IIA Cervical Cancer|Stage IIB Cervical Cancer|Stage IIIA Cervical Cancer|Stage IIIB Cervical Cancer | Drug: Cisplatin|Radiation: External Beam Radiation Therapy|Radiation: Internal Radiation Therapy|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Radiation: Pulsed-Dose Rate Brachytherapy|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 1 | 2013-09-01 | 2017-01-31 |
| NCT02037230 | Adenocarcinoma of the Pancreas | Drug: MK-1775|Drug: Gemcitabine|Radiation: Radiation Therapy | University of Michigan Cancer Center | Phase 1|Phase 2 | 2014-01-01 | 2016-07-01 |
| NCT02508246 | Head and Neck Squamous Cell Carcinoma | Drug: Cisplatin|Drug: Docetaxel|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Procedure: Therapeutic Conventional Surgery|Drug: WEE1 Inhibitor AZD1775 | University of Washington|National Cancer Institute (NCI) | Phase 1 | 2015-07-01 | 2016-11-21 |
| NCT02194829 | Metastatic Pancreatic Adenocarcinoma|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer|Unresectable Pancreatic Carcinoma | Drug: Gemcitabine Hydrochloride|Other: Laboratory Biomarker Analysis|Drug: Paclitaxel Albumin-Stabilized Nanoparticle Formulation|Other: Pharmacological Study|Other: Placebo|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 1|Phase 2 | 2014-07-01 | 2017-01-31 |
| NCT01922076 | Diffuse Intrinsic Pontine Glioma|Untreated Childhood Anaplastic Astrocytoma|Untreated Childhood Anaplastic Oligoastrocytoma|Untreated Childhood Glioblastoma|Untreated Childhood Gliosarcoma | Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Radiation: Radiation Therapy|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 1 | 2013-09-01 | 2017-03-22 |
| NCT01849146 | Adult Glioblastoma|Recurrent Glioblastoma | Other: Laboratory Biomarker Analysis|Other: Pharmacological Study|Radiation: Radiation Therapy|Drug: Temozolomide|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 1 | 2013-08-01 | 2017-03-17 |
| NCT01827384 | Advanced Malignant Solid Neoplasm|Recurrent Malignant Solid Neoplasm | Drug: Carboplatin|Drug: Everolimus|Other: Laboratory Biomarker Analysis|Drug: Temozolomide|Drug: Trametinib|Drug: Veliparib|Drug: WEE1 Inhibitor AZD1775 | National Cancer Institute (NCI) | Phase 2 | 2013-12-01 | 2017-03-23 |
| NCT02341456 | Advanced Solid Tumours | Drug: AZD1775|Drug: Paclitaxel|Drug: carboplatin | AstraZeneca | Phase 1 | 2015-01-01 | 2017-02-21 |
| NCT02272790 | Ovarian, Fallopian Tube, Peritoneal Cancer, P53 Mutation | Drug: AZD1775 + carboplatin|Drug: AZD1775 + PLD | AstraZeneca | Phase 2 | 2015-01-30 | 2017-03-21 |
| NCT02207010 | Glioblastoma|GBM | Biological: AZD1775 | St. Joseph's Hospital and Medical Center, Phoenix|The Ben & Catherine Ivy Foundation|American Society of Clinical Oncology|Barbara Ann Karmanos Cancer Institute|Translational Genomics Research Institute | Early Phase 1 | 2014-07-01 | 2017-01-04 |
| NCT02087241 | Previously Untreated Stage IV Non-Squamous Non Small Cell Lung Cancer | Drug: AZD1775|Drug: AZD1775 Matching Placebo|Drug: pemetrexed|Drug: carboplatin | AstraZeneca | Phase 2 | 2014-03-01 | 2015-06-16 |
| NCT02688907 | Small Cell Lung Cancer | Drug: AZD1775 | Samsung Medical Center | Phase 2 | 2016-04-01 | 2016-02-22 |
| NCT02593019 | Small Cell Lung Cancer | Drug: AZD1775 | Samsung Medical Center | Phase 2 | 2015-12-01 | 2016-02-16 |





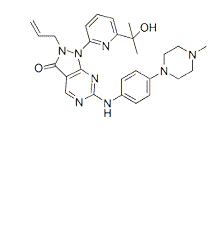
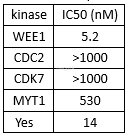
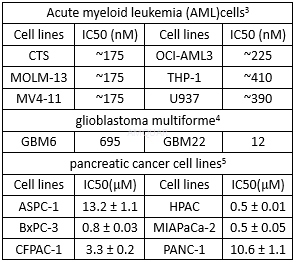
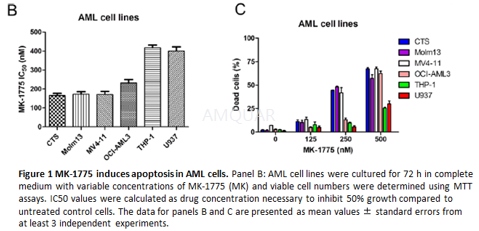
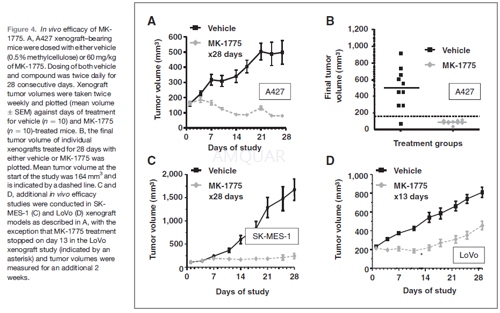 .
.










