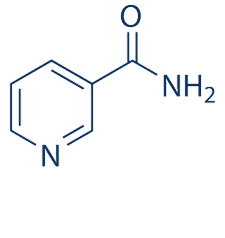
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02213094 | Pregnancy Induced Hypertension|Superimposed Preeclampsia|Hypertension | Drug: Nicotinamide | University of North Carolina, Chapel Hill | Phase 1 | 2013-10-01 | 2016-01-04 |
| NCT02689882 | Metabolic Disturbance | Dietary Supplement: nicotinamide riboside | University of Washington|National Heart, Lung, and Blood Institute (NHLBI) | Phase 1 | 2015-11-01 | 2016-02-18 |
| NCT02416739 | Non-Small-Cell Lung Carcinoma | Drug: Nicotinamide | Il Yeong Park, Ph.D.|Chungbuk National University | Phase 2|Phase 3 | 2015-03-01 | 2016-07-25 |
| NCT01011699 | Chronic Renal Failure|Hemodialysis | Drug: nicotinamide|Drug: sevelamer|Drug: cinacalcet | Centre Hospitalier Universitaire, Amiens | Phase 3 | 2010-01-01 | 2016-05-13 |
| NCT03061474 | Alzheimer's Disease|Mild Cognitive Impairment | Drug: Nicotinamide|Drug: Placebo Comparator | University of California, Irvine | Phase 2 | 2017-03-01 | 2017-02-17 |
| NCT01942291 | Hypoalphalipoproteinemia | Drug: Niacin | University of Campinas, Brazil | Phase 4 | 2012-03-01 | 2013-09-10 |
| NCT01250990 | Dyslipidemias | Drug: Niacin|Other: Placebo | University of Pennsylvania|National Heart, Lung, and Blood Institute (NHLBI) | | 2010-11-01 | 2016-06-24 |
| NCT02018965 | HIV | Drug: Niacin|Drug: Niacin | McGill University Health Center|CIHR Canadian HIV Trials Network | Phase 2 | 2011-11-01 | 2017-03-21 |
| NCT02921659 | Aging | Dietary Supplement: Niagen鈩Dietary Supplement: Placebo | University of Colorado, Boulder|ChromaDex, Inc. | Phase 1|Phase 2 | 2015-04-01 | 2016-11-29 |
| NCT01200160 | Cardiovascular Diseases | Drug: Niacin | Abbott|QUASY | | 2010-02-01 | 2014-05-15 |
| NCT02558595 | Polycystic Kidney Disease | Dietary Supplement: Niacinamide|Other: Placebo | University of Kansas Medical Center|National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | Phase 2 | 2015-09-01 | 2016-11-30 |
| NCT00580931 | Alzheimer's Disease | Drug: Nicotinamide|Drug: Enduramide placebo | University of California, Irvine|Alzheimer's Association | Phase 1|Phase 2 | 2008-01-01 | 2017-01-23 |
| NCT01763424 | Psoriasis | Drug: Calcipotriol plus Nicotinamide|Drug: Calcipotriol | Isfahan University of Medical Sciences | Phase 2|Phase 3 | 2011-07-01 | 2013-01-05 |
| NCT01321034 | Hypercholesterolemia | Drug: Niacin/Laropiprant | Instituto Aragones de Ciencias de la Salud|Hospital Miguel Servet | Phase 4 | 2011-10-01 | 2013-01-08 |
| NCT02950441 | Aging | Dietary Supplement: Nicotinamide Riboside|Other: Placebo | University of Birmingham | Early Phase 1 | 2016-06-01 | 2016-11-04 |
| NCT01589809 | Neurodegenerative Disorders | Drug: nicotinamide | Imperial College London | Phase 2 | 2012-06-01 | 2017-01-17 |
| NCT02835664 | Obesity|Insulin Resistance | Dietary Supplement: Nicotinamide Riboside (Niagen)|Dietary Supplement: Placebo | Maastricht University Medical Center|Dutch Heart Foundation | | 2016-12-01 | 2016-12-21 |
| NCT02836184 | Hyperphosphatemia | Drug: Nicotinic Acids|Drug: Calcium Carbonate | Jiujiang No.1 People's Hospital | Phase 4 | 2016-07-01 | 2016-08-29 |