-
生物活性
Doxorubicin is an inhibitor of Reverse transcriptase and RNA Polymerase.Highly effective myotoxin that inhibits topoisomerase II. Binds to Nucleic acids, presumably by specific intercalation into the DNA double helix, thereby inhibiting necleic acid synthesis. Induces apoptosis.
The cell viability of doxorubicin hydrochloride
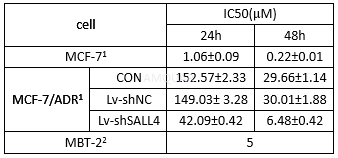
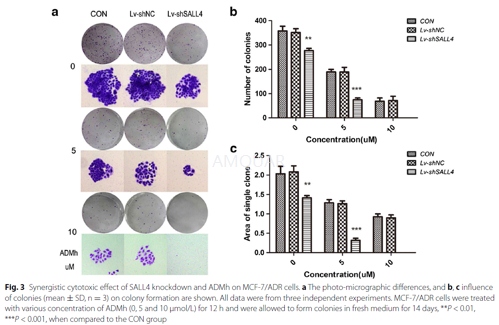
CON: blank control group, Lv-shNC: the negative control group, Lv-shSALL4: recombinant lentiviruses expressing SALL4-shRNA.
Effect of Doxorubicin on Ca2+channel[3]

The cell viability of doxorubicin
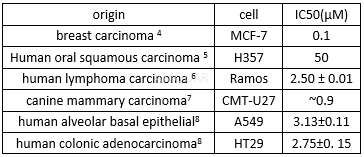
Differentcell viability of doxorubicin in3D spheroids and traditional 2D cell culture systems[9]
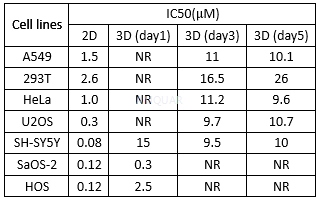
NR: IC50 not reacher.
Doxorubicininhibits DNA topoisomerase I (Topo I) activity at an IC50 value of 0.8μM.[10]
-
体外研究
-
体内研究
dissolve in DMSO,then dillute in Normal saline(生理盐水)
-
激酶实验
Assay of DNA Topo I activity[10]
Topo I (0.1 mg protein/ml) purified from YEpGALI-hTOP1 was diluted 1 : 10 in 70mM potassium phosphate (pH 7.5), 0.5mM dithiothreitol, 0.5 mM ethylenediaminetetraacetic acid (EDTA), and 50% glycerol and was then incubated in 25μl reactions containing 20mM TRIS-HC1 (pH 7.5), 150mM KCl, 10mM EDTA, 1mM 2-mercaptoethanol, 10% glycerol, and 0.8μg supercoiled pHC624 DNA at 30oC for 60 min. Topo I (16ng proteirdml) purified from HeLa cells was diluted 1 : 10 in 100mM potassium phosphate (pH 7.0), 5mM dithiothreitol, 1mM EDTA, 1mM sodium metabisulfite, 1mM benzamide HCI, 0.2mM phenylmethylsulfonyl fluoride, 1μM leupeptin, 1μM pepstatin A, and 50% glycerol and was then incubated in 25μl reactions containing 50mM TRIS-HC1 (pH 7.5), 50mM KCl, 10mM MgCl2, 1mM EDTA, 5mM dithiothreitol, 30μl bovine serum albumin, and 0.8μg supercoiled pHC624 DNA at 37oC for 30 min. All reactions were terminated by the addition of 0.17mg proteinase K/ml, 0.17% sodium dodecyl sulfate, 21mM EDTA, 0.002% bromophenol blue, and 1.7% glycerol followed by incubation at 37oC for 15 min. DNA was electrophoresed on 1% agarose submarine gels in 80mM TRIS-acetate (pH 8.3), 2mM EDTA, and 5μg ethidium bromide/ml at 33 V for 4 h with circulation of the reservoir buffer. DNA bands were visualized under illumination from a shortwave UV light and were photographed with a Polaroid MP-3 camera using Polaroid type 55 film. The developed negatives were scanned with a Hoefer GS 300 scanning microdensitometer, and the areas under the peaks were determined using Hoefer Gelscan software. One unit of DNA topoisomerase activity is defined as the amount of enzyme activity that converts 50% of dimeric pHC624 supercoiled DNA to relaxed DNA under standard reaction conditions.

-
细胞实验
Cell lines and culture[1]
The human breast cancer cell lines, MCF-7, MDAMB-231, SK-BR-3, ZR-75-1 and the human mammary epithelial cell line, HBL-100 and human multidrug resistant breast cancer cell line,
MCF-7/ADR, was obtained. HBL-100 was cultured in RPMI-1640 medium supplemented with 10 % fetal bovine serum (FBS) as well as 1 % penicillin–streptomycin. The other cells were all cultured in Dulbecco modified Eagle medium (DMEM), supplemented with 10 % FBS and 1 % penicillin–streptomycin. Cells were all passaged by trypsinization every 2–3 days and maintained at37 °C in 5 % CO2.
Drugs
Doxorubicin hydrochloride (ADMh) and Verapamil were dissolved in Strokephysiological saline solution to create a stock solution (10 mmol/L), which was stored at −20 °C. To prepare working solutions, the stock solution was further diluted with culture media to yield the desired ADMh concentration.
Colony formation assay
Cells in the logarithmic growth phase were aliquoted as single cell suspension and 400 cells were placed into each well of 6-well plates. After adherence, cells were treated with ADMh (5 and10 umol/L) for 12 h. Then, all plates were incubated for14 days to allow colony formation. Next, cells were fixed with 4 % paraformaldehyde and stained with 0.1 % crystal violet for 20 min. After washing, the plates were air-dried, and stained colonies were photographed with a microscope. The total number of colonies (>50 cells/colony) was counted.
Cell lines and culture[2]
MBT-2 murine bladder cancer cells were grown in RPMI1640 medium supplemented with 10% fetal calf serum. Cells were maintained at 37OC in an incubator with a 5% CO2atmosphere. All experiments were performed with cells in log phase growth. In experiments involving the addition of peroxide to cells peroxide was added to the medium to its final concentration before transfer of the medium to the cell cultures.
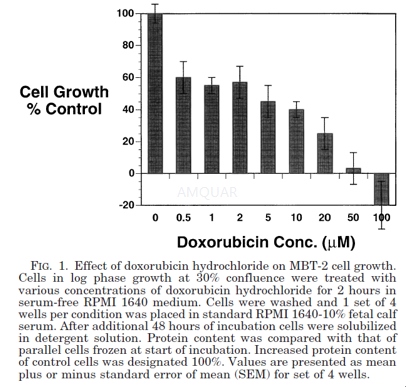
The effect of doxorubicin hydrochloride on cell growth
MBT-2 cells were plated at a density of approximately 15% of confluence in 6-well culture dishes. Cells were allowed to recover from trypsin treatment for 24 hours before any experimental manipulation. Doxorubicin hydrochloride was dissolved in 50 mM Hepes buffer solution, pH 7.2. Drug concentration was determined from its extinction coefficient by measuring absorbance at 480 nm. A stock solution of 2.5 mM doxorubicin hydrochloride was used for all additions to cells in culture. Doxorubicin hydrochloride stock was made fresh for each experiment to minimize agent degradation.
At the initiation of doxorubicin hydrochloride treatment cells from 3 wells were frozen to provide material for determining baseline cellular protein content. Doxorubicin hydrochloride was added to the remaining wells in graded concentrations except for control cells for 2 hours. This time of drug exposure was chosen to simulate the maximum time that the agent would be retained in the bladder by patients treated with intravesical therapy. At the end of this time, medium containing the doxorubicin hydrochloride was removed and replaced by standard medium. Cells were then allowed to grow for 2 additional days. Medium was removed and the wells were washed with phosphate buffered saline (PBS) to eliminate debris and dead cells. Cells were then solubilized with 1 ml 0.2% Triton X-100, 0.15 M NaCl and 0.02 M tris-Cl, pH 7.2 (Triton-TBS) in the cold for 30 minutes. Nuclei and insoluble material were removed from the samples by centrifugation for 5 minutes in an Eppendorf microcentrifuge. Supernatant from this step was assayed for protein using the BioRad protein assay kit. Baseline cell samples that had been frozen at the start of the experiment were solubilized and assayed in similar fashion. Increased protein content of control cells over that of baseline cells was designated as 100% growth and growth of the other cell samples was referenced to this value.
-
动物实验
Animals and administration[11]
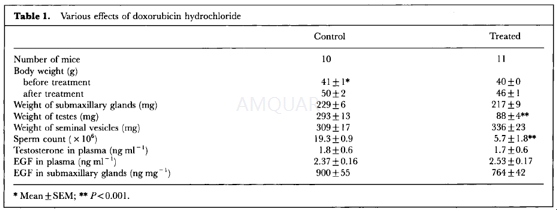
Ten-week old, male ddY mice (body weight, 41±1 g) were used. They were injected subcutaneously with doxorubicin hydrochloride at a dose of 0.3 mg/kg three times a week for 5 weeks. Control animals received the same volume of saline. Four days after the last injection, all mice were bled to death under ether anaesthesia, and their testes, seminal vesicles, and submaxillary glands were weighed. The blood samples were chilled immediately to 4°C and centrifuged, and the plasma was stored at -20oC before being assayed. Submaxillary glands were homogenized with a Polytron homogenizer with 5 ml of cold distilled water added per g of wet tissue. The homogenate was centrifuged at 16,000g for 10 min at 4oC. Portions of the supernatant were sampled and stored at -20oC until use. The testes were fixed in Bouin's fluid for histological examination. One epididymis was isolated and freed of blood and adipose tissue.
Spermatozoa were obtained from the minced caudae in 15 ml of saline
Hormone assays
Plasma testosterone levels were measured by radioimmunoassay (RIA) with solvent partitioning.
Testosterone was extracted with 3 ml of 2: 5 (v/v) mixture of benzene and petroleum ether; its recovery was 76%. This assay used a rabbit antibody raised against testosterone-7α-carboxymethyl-thioether-bovine serum albumin. Cross-reactivity was 100% with testosterone, 23% with 5α-dihydrotestosterone, 1.7% with androstenedione, and 0.2% with dehydroepiandrosterone. The sensitivity of the assay and the intra- and interassay coefficients of variation were 6.25pg per tube, 3.4%, and 12.5%, respectively. The EGF levels in plasma and tissues were measured using an RIA kit for mouse EGF (mouse EGF reagent pack for RIA). This assay used a rabbit antibody raised against mouse EGF. Crossreactivity was 100% with mouse EGF, 0.003% with human EGF, and 100% with rat-transforming growth factor-α. The sensitivity of the assay and the intra- and inter-assay coefficients of variation were 0.4ng per tube, 6.5%, and 8.4%, respectively.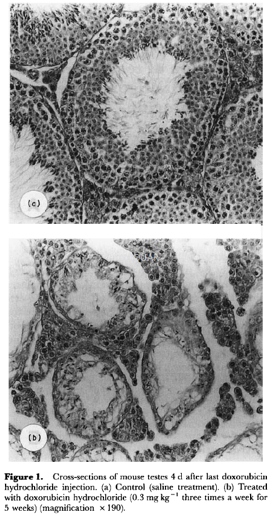
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Chen Y-Y, Li Z-Z, Ye Y-Y, et al. Knockdown of SALL4 inhibits the proliferation and reverses the resistance of MCF-7/ADR cells to doxorubicin hydrochloride. BMC Molecular Biology. 2016;17(1).
[2] Loughlin KR MK, Cragnale D, Wilson L, Ball RA, Bridges KR. The use of hydrogen peroxide to enhance the efficacy of doxorubicin hydrochloride in a murine bladder tumor cell line. J Urol. . 2001;165(4):1300-1304.
more
分子式
C27H29NO11.HCl |
分子量
579.98 |
CAS号
25316-40-9 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
>10 mg/mL |
Water
50 mM |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00969462 | Non-Hodgkin's Lymphoma | Drug: Doxorubicin | Meir Medical Center | Phase 4 | 2009-09-01 | 2013-06-27 |
| NCT01440088 | Soft Tissue Sarcoma | Drug: TH-302 in Combination with Doxorubicin|Drug: Doxorubicin | Threshold Pharmaceuticals|Sarcoma Alliance for Research through Collaboration (SARC) | Phase 3 | 2011-09-01 | 2016-06-01 |
| NCT01095926 | Wilms Tumor|Neuroblastoma|Soft Tissue Sarcoma|Acute Lymphoblastic Leukemia | Drug: doxorubicin | University Hospital Muenster | Phase 2 | 2010-05-01 | 2013-06-27 |
| NCT02475772 | Ovarian Cancer | Drug: Cisplatin and doxorubicin|Procedure: Cisplatin and doxorubicin | Clemens Tempfer|Ruhr University of Bochum | Phase 1 | 2016-11-01 | 2016-11-13 |
| NCT00878800 | Dose Escalation: Solid Tumors|MTD: Soft Tissue Sarcomas | Drug: PXD101|Drug: Doxorubicin | Onxeo|Spectrum Pharmaceuticals, Inc | Phase 1|Phase 2 | 2006-12-01 | 2015-07-07 |
| NCT01227941 | Ovarian Cancer | Drug: MK-4827 + pegylated liposomal doxorubicin|Drug: MK-4827 + pegylated liposomal doxorubicin | Tesaro, Inc. | Phase 1 | 2010-11-01 | 2016-10-18 |
| NCT01163903 | Advanced Solid Tumours | Drug: pantoprazole sodium for injection|Drug: doxorubicin hydrochloride injection | University Health Network, Toronto|Princess Margaret Hospital, Canada | Phase 1 | 2010-07-01 | 2015-07-14 |
| NCT02562378 | Breast Cancer | Drug: Trastuzumab and non-pegylated liposomal doxorubicin | MedSIR|Roche Pharma AG|Experior | Phase 1 | 2015-10-01 | 2016-11-07 |
| NCT00659178 | Neoplasms, Ovarian | Drug: SB-485232 (interleukin 18), pegylated liposomal doxorubicin | GlaxoSmithKline | Phase 1 | 2008-06-01 | 2016-11-30 |
| NCT02131506 | HER-2 Positive Breast Cancer|Malignant Neoplasm of Breast | Drug: Lapatinib, Caelyx | Istituto Scientifico Romagnolo per lo Studio e la cura dei Tumori | Phase 1 | 2009-12-01 | 2016-02-01 |
| NCT00350948 | Ovarian Neoplasms | Drug: Telcyta|Drug: Liposomal Doxorubicin | Telik | Phase 3 | 2006-05-01 | 2013-11-26 |
| NCT02192021 | Cutaneous T Cell Lymphoma | Drug: Micro needle array-Doxorubicin (MNA-D) | University of Pittsburgh | Phase 1 | 2016-01-01 | 2016-09-23 |
| NCT00581360 | Adenoid Cystic Carcinoma | Drug: doxorubicin and bortezomib | University of Pittsburgh|Millennium Pharmaceuticals, Inc. | Phase 2 | 2007-11-01 | 2016-09-19 |
| NCT02237690 | Ovarian Cancer | Drug: Doxorubicin hydrochloride liposome | Shanghai Fudan-Zhangjiang Bio-Pharmaceutical Co.,Ltd. | Phase 1 | 2014-08-01 | 2016-10-08 |
| NCT03002805 | Sarcoma | Drug: CBT-1庐 | Sarcoma Alliance for Research through Collaboration|CBA Research | Phase 1 | 2017-01-01 | 2017-01-25 |
| NCT02420847 | Bladder Cancer | Drug: Ixazomib|Drug: Gemcitabine|Drug: Doxorubicin | M.D. Anderson Cancer Center|Millennium Pharmaceuticals, Inc. | Phase 1|Phase 2 | 2015-07-01 | 2016-12-27 |
| NCT00949325 | Sarcoma | Drug: temsirolimus (Torisel) plus liposomal doxorubicin (Doxil) | Sidney Kimmel Comprehensive Cancer Center|National Comprehensive Cancer Network|Wyeth is now a wholly owned subsidiary of Pfizer | Phase 1|Phase 2 | 2009-09-01 | 2014-11-25 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们