| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00445341 | Lymphoma | Drug: Flavopiridol | National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) | Phase 1|Phase 2 | 2007-01-01 | 2012-10-18 |
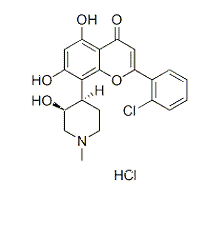
| NCT00058240 | B-cell Chronic Lymphocytic Leukemia|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Waldenstr枚m Macroglobulinemia | Drug: alvocidib | National Cancer Institute (NCI) | Phase 1|Phase 2 | 2003-04-01 | 2014-06-06 |
| NCT00003620 | B-cell Chronic Lymphocytic Leukemia|Refractory Chronic Lymphocytic Leukemia|Stage I Chronic Lymphocytic Leukemia|Stage II Chronic Lymphocytic Leukemia|Stage III Chronic Lymphocytic Leukemia|Stage IV Chronic Lymphocytic Leukemia | Drug: alvocidib|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 1999-06-01 | 2013-01-16 |
| NCT00098371 | B-cell Chronic Lymphocytic Leukemia|Prolymphocytic Leukemia|Refractory Chronic Lymphocytic Leukemia | Drug: alvocidib | National Cancer Institute (NCI) | Phase 2 | 2005-04-01 | 2016-08-31 |
| NCT00047203 | Refractory Multiple Myeloma|Stage I Multiple Myeloma|Stage II Multiple Myeloma|Stage III Multiple Myeloma | Drug: alvocidib | National Cancer Institute (NCI) | Phase 2 | 2002-09-01 | 2013-01-15 |
| NCT00079352 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: alvocidib|Drug: gemcitabine hydrochloride|Drug: irinotecan hydrochloride | National Cancer Institute (NCI) | Phase 1 | 2004-04-01 | 2013-01-24 |
| NCT00047307 | Adenocarcinoma of the Pancreas|Recurrent Pancreatic Cancer|Stage II Pancreatic Cancer|Stage III Pancreatic Cancer|Stage IV Pancreatic Cancer | Drug: alvocidib|Drug: gemcitabine hydrochloride|Radiation: 3-dimensional conformal radiation therapy|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1 | 2002-08-01 | 2013-06-03 |
| NCT00278330 | Blastic Phase Chronic Myelogenous Leukemia|Recurrent Adult Acute Lymphoblastic Leukemia|Recurrent Adult Acute Myeloid Leukemia|Refractory Anemia With Excess Blasts|Relapsing Chronic Myelogenous Leukemia|Untreated Adult Acute Lymphoblastic Leukemia|Untreated Adult Acute Myeloid Leukemia | Drug: alvocidib|Drug: vorinostat | National Cancer Institute (NCI) | Phase 1 | 2006-01-01 | 2013-04-01 |
| NCT00464633 | Leukemia, Lymphocytic, Chronic | Drug: alvocidib | Sanofi | Phase 2 | 2007-03-01 | 2013-02-08 |
| NCT00012181 | Recurrent Childhood Brain Stem Glioma|Recurrent Childhood Cerebellar Astrocytoma|Recurrent Childhood Cerebral Astrocytoma|Recurrent Childhood Ependymoma|Recurrent Childhood Large Cell Lymphoma|Recurrent Childhood Liver Cancer|Recurrent Childhood Lymphoblastic Lymphoma|Recurrent Childhood Malignant Germ Cell Tumor|Recurrent Childhood Medulloblastoma|Recurrent Childhood Rhabdomyosarcoma|Recurrent Childhood Small Noncleaved Cell Lymphoma|Recurrent Childhood Soft Tissue Sarcoma|Recurrent Childhood Supratentorial Primitive Neuroectodermal Tumor|Recurrent Childhood Visual Pathway and Hypothalamic Glioma|Recurrent Childhood Visual Pathway Glioma|Recurrent Ewing Sarcoma/Peripheral Primitive Neuroectodermal Tumor|Recurrent Neuroblastoma|Recurrent Osteosarcoma|Recurrent Retinoblastoma|Recurrent Wilms Tumor and Other Childhood Kidney Tumors|Recurrent/Refractory Childhood Hodgkin Lymphoma|Unspecified Childhood Solid Tumor, Protocol Specific | Drug: alvocidib|Other: pharmacological study | National Cancer Institute (NCI) | Phase 1 | 2001-04-01 | 2013-07-01 |
| NCT00331682 | Adenocarcinoma of the Pancreas|Recurrent Pancreatic Cancer|Stage IV Pancreatic Cancer | Drug: alvocidib|Drug: docetaxel | National Cancer Institute (NCI) | Phase 2 | 2006-03-01 | 2014-05-12 |
| NCT00377104 | B-cell Chronic Lymphocytic Leukemia|Contiguous Stage II Small Lymphocytic Lymphoma|Noncontiguous Stage II Small Lymphocytic Lymphoma|Stage I Chronic Lymphocytic Leukemia|Stage I Small Lymphocytic Lymphoma|Stage II Chronic Lymphocytic Leukemia|Stage III Chronic Lymphocytic Leukemia|Stage III Small Lymphocytic Lymphoma|Stage IV Chronic Lymphocytic Leukemia|Stage IV Small Lymphocytic Lymphoma | Drug: alvocidib|Other: pharmacological study|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1 | 2006-09-01 | 2013-07-01 |
| NCT00083122 | Recurrent Ovarian Epithelial Cancer|Recurrent Primary Peritoneal Cavity Cancer|Stage IIIA Ovarian Epithelial Cancer|Stage IIIA Primary Peritoneal Cavity Cancer|Stage IIIB Ovarian Epithelial Cancer|Stage IIIB Primary Peritoneal Cavity Cancer|Stage IIIC Ovarian Epithelial Cancer|Stage IIIC Primary Peritoneal Cavity Cancer|Stage IV Ovarian Epithelial Cancer|Stage IV Primary Peritoneal Cavity Cancer | Drug: cisplatin|Drug: alvocidib|Drug: cisplatin/flavopiridol | National Cancer Institute (NCI) | Phase 2 | 2004-04-01 | 2014-05-06 |
| NCT00098579 | Gastrointestinal Stromal Tumor|Recurrent Adult Soft Tissue Sarcoma|Stage IV Adult Soft Tissue Sarcoma | Drug: alvocidib|Drug: doxorubicin hydrochloride | National Cancer Institute (NCI) | Phase 1 | 2004-10-01 | 2013-03-18 |
| NCT00324480 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: alvocidib|Drug: vorinostat|Other: pharmacological study | National Cancer Institute (NCI) | Phase 1 | 2006-03-01 | 2014-02-21 |
| NCT00112684 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: alvocidib|Other: pharmacological study|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 1 | 2006-02-01 | 2014-02-21 |
| NCT00080990 | Unspecified Adult Solid Tumor, Protocol Specific | Drug: alvocidib|Drug: fluorouracil|Drug: oxaliplatin|Drug: leucovorin calcium | National Cancer Institute (NCI) | Phase 1 | 2004-02-01 | 2013-12-13 |