-
生物活性
Microtubule stabilization agent that promotes tubulin polymerization and induces G2-M cell cycle arrest (EC50 = 32 nM in HeLa cells). Inhibits proliferation of human carcinoma cell lines in vitro, including MDR cells overexpressing the P-glycoprotein efflux pump. Exhibits potent cytotoxicity in MCF-7 and A549 cells (EC50 values are 0.3 and 2.7 nM respectively). Inhibits growth of HCT-15 tumors in mice in vivo. Additional experiments suggest that Epothilone B causes cell cycle arrest at the G2 stage and eventually leads to cytotoxicity.
The EC0.01 of tubulin is 1.8 μM.[1]
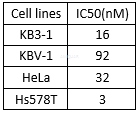
The cytotoxicity of epothilone B in various cancer cell lines and normal cells

The inhibition of mitotic arrest[6]
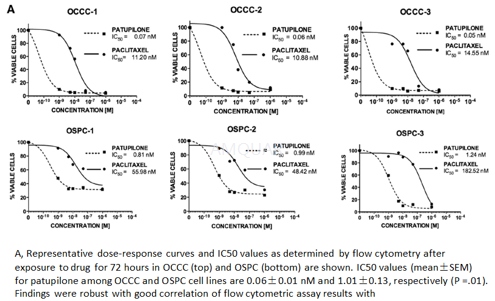
Growth inhibition of ovarian cancer cell lines[7]

OCCC, ovarian clear cell carcinoma; OSPC, ovarian serous papillary carcinomas
Cell growth inhibition of CCRF-CEM and its drug-resistant sublines[8]

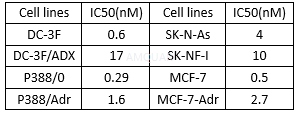
In vitro growth inhibition potency against various parent and drug-resistant tumor cell lines[8]
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% propylene glycol
-
激酶实验
Tubulin polymerization assays
Calf brain microtubule protein (MTP), which includes ~ 15-20% microtubule associated proteins, was purified. The final buffer solution used for all of the drug-protein studies (MES buffer) contained 0.1 M 2-morpholinoethanesulfonic acid (MES), 1 mM ethyleneglycol bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), 0.5 mM MgCl2, and 3 M glycerol at pH 6.6. Samples for electron microscopy were placed on carbon-over-Parlodion-coated grids (300 mesh) and negatively stained with 2% uranyl acetate.
Microtubule assembly in the presence or absence of drugs was monitored spectrophotometrically by using a spectrophotometer equipped with a thermostatically regulated liquid circulator. The temperature was held at 35OC and changes in turbidity (representative of polymer mass) were monitored at 350 nm.
Effective concentration (EC0.01) is defined as the interpolated concentration capable of inducing an initial slope of 0.01 OD/min rate and is calculated using the formula EC0.01=concentration/slope. EC0.01 values are expressed as the mean with standard deviation obtained from three different concentrations.
-
细胞实验
Cell culture[7]
Tumor cells were maintained as a monolayer in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin, and 0.3% amphotericin. Cells were incubated at 37oC in a humidified atmosphere of 95% air/5% CO2.
Characterization of cellular growth rate
Cells at≥80% confluence were harvested by trypinsinization using 0.05% trypsin-EDTA, counted on a hemocytometer, and plated at a density of 100,000 cells/2 mL medium. Cells were incubated as above and trypsinized at 24, 48, 72, and 96 hours. Viable cells were assessed by trypan blue exclusion. Doubling time (DT) was calculated for exponential growth according to DT = (hours elapsed) (ln2)/[ln(number cellst2/number cellst1)].
Viability assays
Patupilone and paclitaxel were dissolved in dimethyl sulfoxide and stored at -20oC protected from light. Cells at log phase of growth were seeded at optimum density and exposed to drug at 24 hours. After 48-72 hours of additional incubation, well contents were harvested in entirety, centrifuged then stained with either trypan blue for manual counts or propidium iodide (2μL of 500μg/mL solution in PBS, 0.1% sodium azide, 2% FBS) for flow cytometric counts. Unless stated otherwise, all IC50 values presented have been determined by flow cytometry.
-
动物实验
Drugs and schedules of administration[9]
Epothilone B solution was prepared before each administration and the drug was dissolved in PEG300/saline (30/70%) and administered intravenously. The study was divided into 2 parts and epothilone B was administered in Experiment 1 to Wistar rats and in Experiment 2 to Fischer rats.
Animals were housed in a limited access animal facility where animal room temperature and relative humidity were set at 22±2 °C and 55±10% respectively. Artificial lighting provided a 24-hour cycle of 12 h light/12 h dark (7 a.m.–7 p.m.).
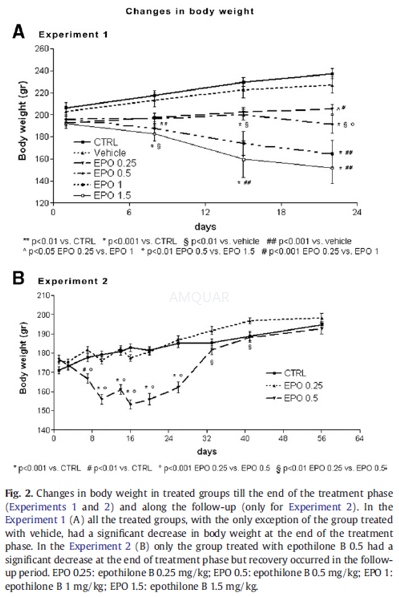
Experiment 1
Fifty-six young adult female Wistar rats (175–200 g on arrival at the housing room) were used; in order to define the Maximum Tolerated Dose (MTD) of the weekly schedule of the selected doses of epothilone B, 5 groups of rats (n=8/group) were treated with 0.25, 0.5, 1, 1.5 or 2 mg/kg weekly×4 weeks. Two groups of control rats (n=8 rats/group) were represented by untreated animals and by rats treated with epothilone B vehicle (PEG300/saline).
Experiment 2
60 young adult female Fischer rats (175–200 g on arrival at the housing room) were treated with epothilone B 0.25, 0.5, 1 or 1.5 mg/kg weekly×4 weeks (n=12 rats/group). A group of control rats was represented by 12 untreated animals. Part of the rats from this experiment underwent a 4-week follow-up period of observation after drug treatment withdrawal.
Clinical signs and body weight
The general clinical condition of the animals was assessed daily during treatment and the follow-up periods. Body weight was recorded before each administration, on the days of scheduled sacrifices and at the 4-week final evaluation at the end of the follow-up period.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Alicia Regueiro-Ren RMB, Xiaoping Zheng, Soong-Hoon Kim,, James A. Johnson CRF, Francis Y. F. Lee, Byron H. Long, and, Vite GD. Synthesis and Biological Activity of Novel Epothilone Aziridines. Org Lett. 2001;3(17):2693-2696.
[2] Pagano A, Honore S, Mohan R, Berges R, Akhmanova A, Braguer D. Epothilone B inhibits migration of glioblastoma cells by inducing microtubule catastrophes and affecting EB1 accumulation at microtubule plus ends. Biochem Pharmacol. 2012;84(4):432-443.
more
分子式
C27H41NO6S |
分子量
507.68 |
CAS号
152044-54-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
110 mg/mL |
Water
<1 mg/mL |
Ethanol
100 mg/mL |
体内溶解度
5 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00450866 | Breast Cancer|Metastatic Cancer | Biological: epothilone B | David Peereboom, MD|National Cancer Institute (NCI)|Case Comprehensive Cancer Center | Phase 2 | 2007-01-01 | 2014-01-28 |
| NCT00412789 | Tumors | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 1 | 2006-08-01 | 2010-05-28 |
| NCT00420615 | Solid Tumors | Drug: Patupilone and Omeprazole|Drug: Patupilone + Midalzolam | Novartis Pharmaceuticals|Novartis | Phase 1 | 2006-12-01 | 2012-04-23 |
| NCT00035100 | Ovarian Neoplasms|Peritoneal Neoplasms|Fallopian Tube Neoplasms | Drug: epothilone b | Novartis Pharmaceuticals|Novartis | Phase 2 | 2001-09-01 | 2012-04-13 |
| NCT00035165 | Melanoma | Drug: epothilone b | Novartis Pharmaceuticals|Novartis | Phase 2 | 2002-03-01 | 2012-04-24 |
| NCT00050349 | Carcinoid|Neuroendocrine Tumors | Drug: EPO906 epothilone B | Novartis Pharmaceuticals|Novartis | Phase 2 | 2002-07-01 | 2017-02-28 |
| NCT00273312 | Hepatocellular Carcinoma | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 2 | 2006-01-01 | 2017-02-03 |
| NCT00171834 | Carcinoma, Non-Small-Cell Lung | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 1|Phase 2 | 2003-08-01 | 2014-01-06 |
| NCT00262990 | Ovarian Cancer|Fallopian Tube Cancer|Peritoneal Neoplasms | Drug: EPO906 (Patupilone)|Drug: doxorubicin | Novartis Pharmaceuticals|Novartis | Phase 3 | 2005-11-01 | 2012-05-02 |
| NCT00035243 | Kidney Neoplasms | Drug: epothilone b | Novartis Pharmaceuticals|Novartis | Phase 2 | 2002-04-01 | 2012-04-16 |
| NCT00448396 | Advanced Malignancies | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 1 | 2007-03-01 | 2009-11-18 |
| NCT00159484 | Colon Cancer|Colorectal Cancer | Drug: EPO906, celecoxib | University of Southern California|Novartis | Phase 1|Phase 2 | 2004-10-01 | 2015-03-25 |
| NCT00426582 | Advanced Solid Tumors | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 1 | 2006-08-01 | 2011-03-24 |
| NCT00426140 | Advanced Malignancies|Solid Tumors | Drug: Patupilone | Novartis Pharmaceuticals|Novartis | Phase 1 | 2006-08-01 | 2010-04-22 |
| NCT00442741 | Solid Tumors | Drug: Patupilone|Drug: Patupilone + Omeprazole | Novartis Pharmaceuticals|Novartis | Phase 1 | 2007-07-01 | 2012-04-23 |
| NCT00328458 | Central Nervous System Neoplasms|Head and Neck Neoplasms | Drug: EPO906 (epothilone B) | Thomas Jefferson University|Novartis Pharmaceuticals | Phase 1 | 2004-02-01 | 2011-03-11 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们