-
生物活性
Niclosamide is inhibitor of the STAT3 signaling pathway; inhibits the activation, nuclear translocation and transactivation of STAT3. Displays selectivity for STAT3 over STAT1, STAT5, JAK1, JAK2 and Src kinases. Inhibits the transcription of STAT3 target genes and induces cell growth inhibition, apoptosis and cell cycle arrest of cancer cells with constitutively active STAT3.
Niclosamide dose dependently inhibits STAT3-dependent luciferase reporter activity with an IC50 of 0.25±0.07μM in HeLa cells.[1]
Niclosamide significantly inhibits the proliferation of CML cell lines with an ED50 of 6-7μM.[2]
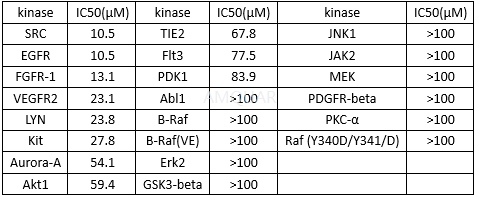
IC50 of Niclosamide against 22 protein kinases[1]
Selectively anti-proliferation of the cancer cells[1]
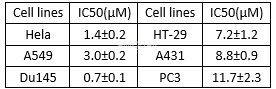
IC50 doses of niclosamide on human cancer cell lines

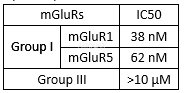
Selectivity of Niclosamide against human recombinant metabotropic glutamate receptor (mGluR)[5]
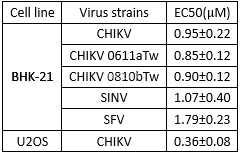
Antiviral activity of Niclosamide against various alphaviruses in vitro[6]
-
体外研究
-
体内研究
1% DMSO+30% polyethylene glycol+1% Tween 80
-
激酶实验
Protein Kinase profiling assay[1]
Protein kinases were purified by affinity chromatography using either GSH-agarose or Ni-NTH-agarose. A radiometric protein kinase assay was used for measuring the kinase activity of the 22 protein kinases. Briefly, for each protein kinase, 50μl reaction cocktail containing 60 mM HEPES-NaOH, 3mM MgCl2, 3mM MnCl2, 3μM Na-orthovanadate, 1.2mM DTT, 50μg/ml PEG20000, 1 μM [γ-33P]-ATP(appox.6×1005cpm), test compound, adequate amount of enzyme and its substrate. The PKC-alpha assay additionally contained 1mM CaCl2, 4mM EDTA, 5μg/ml phosphatidylserine and 1μg/ml 1, 2-Dioleyl-glycerol). The reaction cocktails were incubated at 37oC for 60minutes and stopped with 50μl 2% (v/v) H3PO4. Incorporation of 33Pi was determined with a microplate scintillation counter. The activities and the IC50 values were calculated using Quattro Workflow V2.28
-
细胞实验
Cytotoxicity screening (MTT assay)[7]
Cell viability was determined by MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) colorimetric assay. Briefly, Neuro 2a, A549 and HepG 2 cells were grown in tissue culture flask supplemented with DMEM, EMEM and RPMI medium with 10% fetal bovine serum and antibiotic—antimycotic solution respectively. After reaching 90% confluency, cells were trypsinised, counted and seeded at a density of 5000 cells per well in 96-wellplate and were incubated in 5% CO2incubator. After 24 h incubation, cells were treated with different concentrations (3.125–100μM) ofniclosamide(NCM)and further incubated for 48h. After incubation, the media was removed, fresh media with 100μL of MTT at 0.5 mg/mL concentration was added to each well and incubated for 4 h, form azan crystals were formed and dissolved by adding 200μL of DMSO. The plates were then kept at room temperature for 30 min and absorbance was measured at 570 nm using multi detection plate reader.
-
动物实验
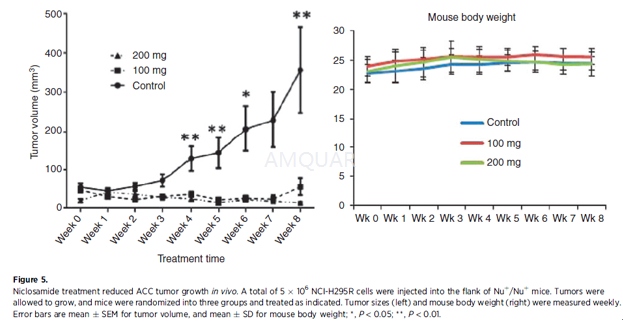
In vivo mouse studies[4]
Mice were maintained according to NIH Animal Research Advisory Committee guidelines. A total of 5 x 106NCI-H295R cells were injected into the flank of Nu+/Nu+mice. Tumors were allowed to grow and mice were randomized into three treatment groups (8 mice per treatment group). Mice were treated with 100 mg/kg of niclosamide, 200 mg/kg of niclosamide, or the vehicle (PEG500) everyday by oral gavage. Tumor sizes were measured in two dimensions every week with calipers and recorded.
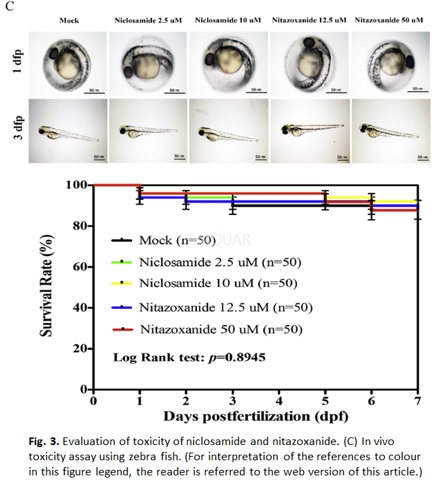
In-vivo zebrafish assays for toxicity testing[6] A dissecting microscope was used to observe morphological anomalies in zebrafish, including chorion with attached debris, delayed development, lack of spontaneous movement, pericardial edema, yolk sac edema, bent trunk, tail malformation, and uninflated swim bladder. We also recorded hatch and survival rates, which are expressed as the number of that have hatched or dead embryos as compared with the control group. We stored 4-5 h post fertilization (hpf) embryos in 48-well plates at a concentration of two embryos/well, and each well contained 1 ml of test solution (i.e. either niclosamide at 2.5 or 10μM, or nitazoxanide at 50 or 12.5μM). Specifically, embryos were stored at 28OC for 7 days (with a 14/10 h light/dark photoperiod) and were updated daily. After experiments were completed, zebrafish were sacrificed using 0.5% tricaine, whereby all efforts were made to minimize suffering.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Ren X, Duan L, He Q, et al. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med Chem Lett. 2010;1(9):454-459.
[2] Liu Z, Li Y, Lv C, Wang L, Song H. Anthelmintic drug niclosamide enhances the sensitivity of chronic myeloid leukemia cells to dasatinib through inhibiting Erk/Mnk1/eIF4E pathway. Biochem Biophys Res Commun. 2016;478(2):893-899.
more
分子式
C13H8Cl2N2O4 |
分子量
327.12 |
CAS号
50-65-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
<1 mg/mL |
Water
<1 mg/mL |
Ethanol
25 mM |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02687009 | Colon Cancer | Drug: Niclosamide | Michael Morse, MD|Duke University | Phase 1 | 2016-07-01 | 2016-05-12 |
| NCT02532114 | Hormone-Resistant Prostate Cancer|Metastatic Prostate Carcinoma|Recurrent Prostate Carcinoma|Stage IV Prostate Adenocarcinoma | Drug: Enzalutamide|Other: Laboratory Biomarker Analysis|Drug: Niclosamide|Other: Pharmacological Study | University of Washington|National Cancer Institute (NCI) | Phase 1 | 2015-12-01 | 2016-12-08 |
| NCT02519582 | Colorectal Cancer | Drug: Niclosamide | Charite University, Berlin, Germany|Center for Molecular Medicine | Phase 2 | 2015-08-01 | 2015-08-06 |
| NCT02807805 | Metastatic Prostate Carcinoma|Recurrent Prostate Carcinoma|Stage IV Prostate Cancer | Drug: Abiraterone Acetate|Drug: Niclosamide|Drug: Prednisone | University of California, Davis|National Cancer Institute (NCI) | Phase 2 | 2016-09-01 | 2016-09-08 |
| NCT00138359 | Intestinal Parasitism | Drug: Niclosamide | National Institute of Allergy and Infectious Diseases (NIAID) | | 2004-12-01 | 2010-08-26 |
| NCT01296958 | Taenia Solium Taeniasis | Drug: Niclosamide|Behavioral: Community education campaign | Oregon Health and Science University|Universidad Nacional Mayor de San Marcos|National Institute of Neurological Disorders and Stroke (NINDS) | | 2011-05-01 | 2014-10-22 |
| NCT01189903 | Asian Colorectal Cancer Patients | Drug: Regorafenib | National University Hospital, Singapore | Phase 2|Phase 3 | 2011-01-01 | 2014-01-26 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们