-
生物活性
AZD5363 inhibits all AKT isoforms with a potency of 10 nmol/L or less and inhibits phosphorylation of AKT substrates in cells with a potency of approximately 0.3 to 0.8 μmol/L. AZD5363 monotherapy inhibits the proliferation of 41 of 182 solid and hematologic tumor cell lines with a potency of 3 μmol/L or less.
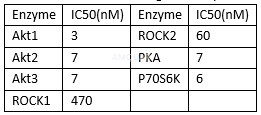
Akt enzyme, cell potency and hERG Activity[1]

*Inhibition of phosphorylation of GSK3β mediated by Akt in MDAMB468 cells,#CHO cells, Ion Works assay.
IC50 values of AZD5363 against enzyme and cellular endpoints[2]
Inhibition of AKTsubstrates in cells[2]

Effect of AZD5363 on cell growth of breast cancer cell lines and their LTED derivatives[3]

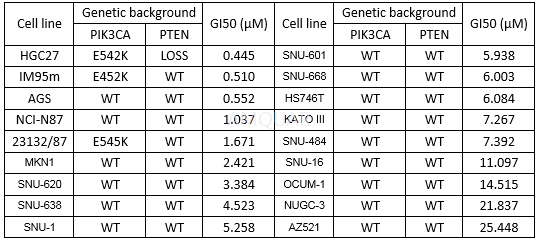
AZD5363 inhibits proliferation of a subset of Gastric cancer (GC) cell lines in vitro[4]
-
体外研究
-
体内研究
溶于 10% DMSO 25% w+v Kleptose HPB
-
激酶实验
Enzyme assays[2]
The ability of AZD5363 to inhibit the activity of AKT1, AKT2, and AKT3 was evaluated by the Caliper Off-Chip Incubation Mobility Shift assay. Active recombinant AKT1, AKT2, or AKT3 were incubated with a 5-FAM–labeled custom-synthesized peptide substrate together with increasing concentrations of inhibitor. Final reactions contained 1 to 3 nmol/L AKT1, AKT2, or AKT3 enzymes; 1.5μmol/L peptide substrate; ATP at Km for each AKT isoform; 10 mmol/L MgCl2, 4 mmol/L dithiothreitol (DTT), 100 mmol/L HEPES, and 0.015% Brij-35. The reactions were incubated at room temperature for 1 hour and stopped by the addition of buffer containing 100 mmol/L HEPES, 0.015% Brij-35 solution, 0.1% coating reagent, 40 mmol/L EDTA, and 5% DMSO. Plates were then analyzed using a Caliper LC3000, allowing for separation of peptide substrate and phosphorylated product by electrophoresis with subsequent detection and quantification of laserinduced fluorescence.
To determine the kinase selectivity profile, AZD5363 was also tested against PKA, ROCK1, ROCK2, and P70S6K. PKA, ROCK1, and ROCK2 activity were determined by Caliper Off-Chip Incubation Mobility Shift Assay, as described earlier. Final reaction conditions for measuring ROCKI activity were 5 nmol/L active recombinant ROCK1 (Invitrogen), 1.5μmol/L fluorescein isothiocyanate (FITC)-labeled custom peptide substrate, 7μmol/L ATP, 1 mmol/L DTT, 5 mmol/L MgCl2, 100 mmol/L HEPES, 0.015% Brij-35, and 5 mmol/Lβ-glycerophosphate; final reaction for measuring ROCK2 activity contained 7.5 nmol/L active recombinant ROCK2, 1.5μmol/L FAM-labeled custom peptide substrate, 7.5μmol/L ATP, 1 mmol/L DTT, 10 mmol/L MgCl2, 100 mmol/L HEPES, 0.015% Brij-35, and 5 mmol/Lβ-glycerophosphate; and protein kinase A (PKA) activity was measured in a final reaction containing 0.0625 nmol/L PKA, 3μmol/L FITClabeled custom peptide substrate, 4.6 mmol/L ATP, 1 mmol/L DTT, 10 mmol/L MgCl2, 110 mmol/L HEPES, and 0.015% Brij-35. P70S6K activity was measured by a radioactive (33P-ATP) filter-binding assay. Recombinant S6K1 (T412E) was assayed against a substrate peptide (KKRNRTLTV) in a final volume of 25.5μL containing 8 mmol/L MOPS, 200μmol/L EDTA, 100μmol/L substrate peptide, 10 mmol/L magnesium acetate,
20μmol/Lγ-33P-ATP (50–1,000 cpm/pmol), and increasing concentrations of AZD5363. The reactions were incubated for 30 minutes at room temperature and terminated by the addition of 0.5 mol (3%) orthophosphoric acid. Reactions were then harvested onto a P81 UniFilter and product formation quantified. IC50 values for all enzyme assays were obtained by fitting data in Origin 7.0.
-
细胞实验
Cells culture[2]
Mouse embryonic fibroblast cells(wild-type,GSK3α-/-, or GSk3β-/-gene knockout) were maintained in a 37oC,5% CO2humidified incubator. All of these cell lines express IL-17 receptors A and C. Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin was used as the growth medium.
Treatment of cells
Mouse embryonic fibroblast cells were seeded in to 60-mm cell culture dishes with 0.5x106cells/ dish. After 4h incubation, the cells were incubated with serum-free DMEM for 20h, and then treated with IL-17, insulin, IGF1 and/ or AZD5363.
Western blot analysis
Following the treatment of cells, proteins were extracted by using RIPA lysis buffer, which contains 50mM sodium fluoride, 0.5% Igepal CA-630, 10mM sodium phosphate, 150mM sodium chloride, 25mM Tris(pH 8.0), 1mM phenylmethylsulfonyl fluoride, 2mM ethylenediamine- tetraacetic acid (EDTA), and 1.2mM sodium vanadate. Protein concentration was assessed by using Bio-Rad Protein Assay Dye Reagent Concentrate and BioTek ELx800 microplate reader. Eighty microgram of protein of each group was loaded to 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. Membrane blocking was done using 5% non-fat dry milk in TBST buffer (25mM Tris-HCl, 125mM sodium chloride, and 0.1% Tween 20). Primary antibody was incubated with the membrane at 4oC overnight. The membrane was washed three times with TBST, and incubated with IRDye® 800CW- or IRDye® 680RD-conjugated secondary antibodies at room temperature for 1 h.

-
动物实验
Human tumor xenografts[3]
In vivo efficacy studies were performed in 8- to 12-week-old female Swiss nude mice implanted with the PDX HBCx22OvaR. First, a pharmacodynamic study was performed for 4 days to assess biomarker changes with samples removed 2 and 4 hours after treatment. Between 5 and 7 mice were included in each group. Second, a long-term study to assess changes in tumor volume and progression over 90 days was initiated. To address the ability of the combination to delay tumor progression, mice were followed for a further 40 days after drug withdrawal. The treatment groups received AZD5363 solubilized in a 10%DMSO 25% w/v Kleptose HPB buffer by oral gavage, or fulvestrant suspended in corn oil by subcutaneous injection into the flank. For the combination groups, fulvestrant was dosed 2 hours before AZD5363. The control groups received both vehicles. In the combination study, treatments were started when tumors reached a volume of 40 to 65 mm3. Each group included between 11 and 13 xenografts. Tumor volumes (measured by caliper), animal body weight, and tumor condition were recorded twice weekly for the duration of the study. Tumor volumes were calculated as V = a X b2/2, where "a" is the largest diameter and "b" is the smallest. Growth inhibition from the start of treatment was assessed by comparison of the differences in tumor volume between control and treated groups. Because the variance in mean tumor volume data increases proportionally with volume (and is therefore disproportionate between groups), data were log-transformed to remove any size dependency before statistical evaluation. Tumor volumes were reported to the initial volume as relative tumor volume (RTV).
Percent of tumor growth inhibition (TGI) was calculated at the end of treatments (day 88) as follow: (1-RTVtreated/RTVcontrol) x 100. Statistical significance was evaluated using a one-tailed, two-sample t test.
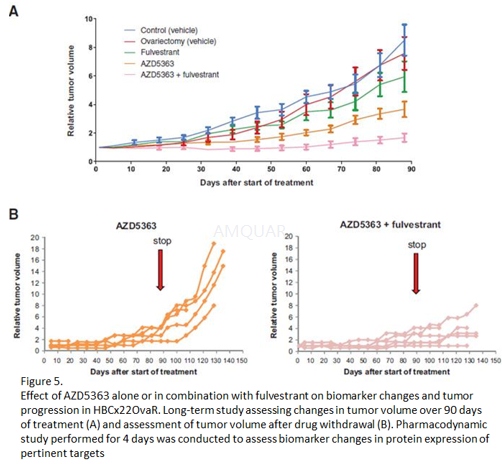
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Addie M, Ballard P, Buttar D, et al. Discovery of 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin -4-yl)piperidine-4-carboxamide (AZD5363), an orally bioavailable, potent inhibitor of Akt kinases. J Med Chem. 2013;56(5):2059-2073.
[2] Davies BR, Greenwood H, Dudley P, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11(4):873-887.
more
分子式
C21H25ClN6O2 |
分子量
428.92 |
CAS号
1143532-39-1 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
82 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02338622 | Advanced Cancer | Drug: olaparib|Drug: AZD5363 | Royal Marsden NHS Foundation Trust|Institute of Cancer Research, United Kingdom|AstraZeneca | Phase 1 | 2014-03-01 | 2015-01-16 |
| NCT02121639 | Prostate Cancer | Drug: Placebo|Drug: AZD5363 | University Hospital Southampton NHS Foundation Trust|AstraZeneca|Cancer Research UK | Phase 1|Phase 2 | 2014-01-01 | 2016-10-12 |
| NCT02208375 | Breast Cancer|Malignant Female Reproductive System Neoplasm | Drug: Olaparib|Drug: AZD2014|Drug: AZD5363 | M.D. Anderson Cancer Center|AstraZeneca|National Cancer Institute (NCI) | Phase 1|Phase 2 | 2014-11-01 | 2017-03-06 |
| NCT01353781 | Advanced Solid Malignancy|Advanced Solid Tumor | Drug: AZD5363 | AstraZeneca | Phase 1 | 2011-06-01 | 2016-04-25 |
| NCT02077569 | Invasive Breast Cancer | Drug: AZD5363 | University of Nottingham|AstraZeneca|Cancer Research UK|National Cancer Research Network | Phase 2 | 2014-01-01 | 2017-02-01 |
| NCT01226316 | Advanced Solid Malignancy|Safety and Tolerability|Pharmacokinetics|Pharmacodynamics|Tumour Response|Advanced or Metastatic Breast Cancer|Ovarian Cancer|Cervical Cancer|Endometrial Cancer|PIK3CA|AKT1|PTEN|ER Positive|HER2 Positive | Drug: AZD5363|Drug: AZD5363|Drug: AZD5363|Drug: AZD5363 | AstraZeneca | Phase 1 | 2010-12-01 | 2017-02-27 |
| NCT01895946 | Advanced Solid Malignancy,|Safety and Tolerability,|Pharmacokinetics, Pharmacodynamics,|Tumour Response, | Drug: AZD5363|Drug: AZD5363 | AstraZeneca | Phase 1 | 2013-12-01 | 2016-04-19 |
| NCT02423603 | Metastatic Breast Cancer | Drug: Paclitaxel|Drug: AZD5363|Drug: Placebo | Queen Mary University of London|AstraZeneca|Cancer Research UK | Phase 2 | 2014-05-01 | 2015-04-17 |
| NCT01625286 | Advanced or Metastatic Breast Cancer|ER+ ve Advanced or Metastatic Breast Cancer | Drug: AZD5363 when combined with weekly paclitaxel.|Drug: AZD5363 when combined with weekly paclitaxel.|Drug: AZD5363when combined with weekly paclitaxel.|Drug: A placebo in combination with weekly paclitaxel. | AstraZeneca | Phase 1|Phase 2 | 2012-10-01 | 2017-01-12 |
| NCT01692262 | Metastatic Castrate-Resistant Prostate Cancer (mCRPC),|Efficacy,|Safety and Tolerability,|Pharmacokinetics,|Pharmacodynamics,|Tumour Response. | Drug: Intermittent dosing of AZD5363|Drug: Intermittent dosing of AZD5363|Drug: Intermittent dosing of AZD5363 | AstraZeneca | Phase 1 | 2012-11-01 | 2014-06-18 |
| NCT02451956 | Advanced Gastric Cancer | Drug: AZD5363|Drug: paclitaxel | Samsung Medical Center | Phase 2 | 2015-01-07 | 2017-02-15 |
| NCT02525068 | Adenocarcinoma of the Prostate | Drug: AZD5363|Drug: Enzalutamide | Institute of Cancer Research, United Kingdom|Royal Marsden NHS Foundation Trust | Phase 2 | 2014-12-01 | 2015-08-13 |
| NCT01992952 | Estrogen Receptor Positive Breast Cancer | Drug: AZD5363|Drug: Placebo|Drug: Fulvestrant | Wales Cancer Trials Unit|Velindre NHS Trust | Phase 1|Phase 2 | 2014-05-01 | 2014-06-10 |
| NCT02449655 | Advanced Gastric Adenocarcinoma | Drug: AZD5363|Drug: paclitaxel|Drug: AZD2014|Drug: paclitaxel | Samsung Medical Center | Phase 2 | 2015-02-12 | 2017-02-10 |
| NCT02576444 | Cancer | Drug: AZD2281|Drug: AZD5363|Drug: AZD1775|Drug: AZD2014 | Joseph Paul Eder|Dana-Farber Cancer Institute|Vanderbilt-Ingram Cancer Center|Yale University | Phase 2 | 2015-11-01 | 2016-11-18 |
| NCT02117167 | Non-small Cell Lung Cancer Metastatic | Drug: AZD2014|Drug: AZD4547|Drug: AZD5363|Drug: AZD8931|Drug: Selumetinib|Drug: Vandetanib|Drug: Erlotinib|Drug: Pemetrexed|Drug: MEDI4736 | UNICANCER|IFCT|Fondation ARC|AstraZeneca | Phase 2 | 2014-04-01 | 2016-02-19 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们
























